Daniel A. Gubler and Dr. Matt A. Peterson, Chemistry and Biochemistry
 The purpose of my research is to synthesize a new class of enediynes that mimic linbenzoadenosine derivatives and have potential for inhibiting HIV integrase. This new class of enediynes is shown in Figure 1. Upon synthesis we will evaluate the biological activity of these compounds.
The purpose of my research is to synthesize a new class of enediynes that mimic linbenzoadenosine derivatives and have potential for inhibiting HIV integrase. This new class of enediynes is shown in Figure 1. Upon synthesis we will evaluate the biological activity of these compounds.
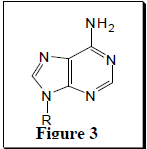
 In 1978 Leonard et al. reported data indicating that linbenzoadenosine derivatives (Figure 2) could fit into the active sites of enzymes requiring adenine derivatives (Figure 3).1 Stretching out Adenine with a benzene ring to form lin-benzoadenosine widens the molecule by 2.4 Å. Despite this widening Leonard et al. showed that lin-benzoadenosine derivatives still exhibit strong enzyme binding and act as cofactors in enzymatic reactions.2 Nair et al. expanded on these findings when in 1998 they showed that the enzyme HIV integrase could be inhibited by novel nucleotides with tricyclic bases such as lin-benzoadenosine.3 HIV integrase is the enzyme responsible for catalyzing the incorporation of viral DNA into the host cell. Without the activity of this enzyme, HIV is not able to replicate and the virus eventually dies. Due to the similarity in structure for compounds 1-6 and lin-benzoadenosine, we postulate that compounds 1-6 might exhibit similar inhibition of HIV integrase.
In 1978 Leonard et al. reported data indicating that linbenzoadenosine derivatives (Figure 2) could fit into the active sites of enzymes requiring adenine derivatives (Figure 3).1 Stretching out Adenine with a benzene ring to form lin-benzoadenosine widens the molecule by 2.4 Å. Despite this widening Leonard et al. showed that lin-benzoadenosine derivatives still exhibit strong enzyme binding and act as cofactors in enzymatic reactions.2 Nair et al. expanded on these findings when in 1998 they showed that the enzyme HIV integrase could be inhibited by novel nucleotides with tricyclic bases such as lin-benzoadenosine.3 HIV integrase is the enzyme responsible for catalyzing the incorporation of viral DNA into the host cell. Without the activity of this enzyme, HIV is not able to replicate and the virus eventually dies. Due to the similarity in structure for compounds 1-6 and lin-benzoadenosine, we postulate that compounds 1-6 might exhibit similar inhibition of HIV integrase.
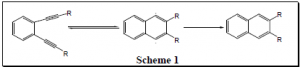 This research is original in that in addition to mimicking lin-benzoadenosine, compounds 1-6 contain an enediyne ring and undergo the Bergman cycloaromatization reaction (B. C.). This reaction is shown in Scheme 1.4 This reaction produces a p-benzyne radical as an intermediate that can oxidatively damage DNA, causing the DNA to unravel, and ultimately bring about cell death.5
This research is original in that in addition to mimicking lin-benzoadenosine, compounds 1-6 contain an enediyne ring and undergo the Bergman cycloaromatization reaction (B. C.). This reaction is shown in Scheme 1.4 This reaction produces a p-benzyne radical as an intermediate that can oxidatively damage DNA, causing the DNA to unravel, and ultimately bring about cell death.5 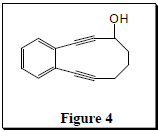 Recent results have shown that 7 (Figure 4) undergoes B.C. at 37 ºC and has a half-life of 6 hours.6 Compounds 4-6 are similar in structure to 7 and are therefore expected to exhibit similar reactivity profiles in B.C. This would be an advantageous property for an antitumor agent. Compounds 1-3 are not expected to undergo B.C. under physiological conditions. In spite of this, it is quite possible that in the active site of an enzyme the distance between the terminal alkyne carbons will be compressed just enough for B.C. to occur. This is novel because the compound would be harmless until it gets into the active site of the enzyme. This feature would eliminate any unwanted reactions of the compound with other substrates before it gets into the active site of an enzyme. As shown above, compounds 1-6 have the potential to be effective drugs both as HIV integrase inhibitors and as antitumor compounds.
Recent results have shown that 7 (Figure 4) undergoes B.C. at 37 ºC and has a half-life of 6 hours.6 Compounds 4-6 are similar in structure to 7 and are therefore expected to exhibit similar reactivity profiles in B.C. This would be an advantageous property for an antitumor agent. Compounds 1-3 are not expected to undergo B.C. under physiological conditions. In spite of this, it is quite possible that in the active site of an enzyme the distance between the terminal alkyne carbons will be compressed just enough for B.C. to occur. This is novel because the compound would be harmless until it gets into the active site of the enzyme. This feature would eliminate any unwanted reactions of the compound with other substrates before it gets into the active site of an enzyme. As shown above, compounds 1-6 have the potential to be effective drugs both as HIV integrase inhibitors and as antitumor compounds.
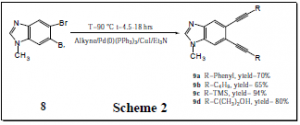
A key step in the synthesis of compounds 1-6 involves converting dibromobenzimidazoles (e.g., 8) to enediynes (e.g., 9a-9d) via a reaction known as the Sonogashira coupling reaction. During the period covered by this grant, I was able to fine tune this reaction and find optimal conditions for obtaining the target compounds in excellent yield. The results of these experiments are shown in Scheme 2. Sonogashira coupling is a known reaction in organic synthesis, but it has not been applied to dibromobenzimidazoles previously. Therefore, optimization of this coupling was a major task, and its successful completion was a significant accomplishment.
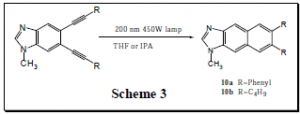
Upon successful synthesis of compounds 9a-9d, we were interested in seeing if these compounds would undergo photochemical B.C. when irradiated with UV light (Scheme 3). It was found that this reaction did proceed to give the desired products (10a-10b), but the products were obtained in very low yield and were difficult to isolate.
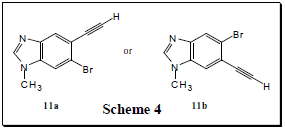
Another recent success was the synthesis of compound 11a or 11b (Scheme 4). We are now in the process of performing nuclear magnetic resonance experiments to determine which of the two compounds we have synthesized. Successful synthesis of 11a/11b takes us a few steps closer to accomplishing our goal of making the major skeleton for compounds 1-6. My time doing research has been an invaluable experience for me and will help me in graduate school and in my career.
References
- Leonard, N. J.; Scopes, D. C.; VanDerLijn, P.; Barrio, J. R. “Dimensional Probes of the Binding Sites of Adenine Nucleotides. Biological effects of Widening the Adenine Ring by 2.4 Å” Biochemistry 1978, 17,
3677–3685. - Leonard, N. J.; Sprecker, M. A.; Morrice, A. G. “Defined Dimensional Changes in Enzyme Substrates and Evaluation of Derivatives of the Benzopurines” J. Am. Chem. Soc. 1976, 98, 3987–3994.
- Zhang, J.; Neamati, N.; Pommier, Y.; Nair, V. “Inhibition of HIV Integrase by Novel Nucleotides Bearing Tricyclic Bases” Bioorg. Med. Chem. Lett. 1998, 8, 1887–1890.
- Jones, R. R.; Bergman R. G. “p-Benzyne. Generation as an Intermediate in a Thermal Isomerization Reaction and Trapping Evidence for the 1,4-Benzenediyl Structure” J. Am. Chem. Soc. 1972, 94, 660–661.
- Borders, D. B.; Doyle T. W.; Eds. “Enediyne Antibiotics as Antitumor Agents”, Marcel Dekker: New York, 1995.
- Boger, D. L.; Zhou, J. “CDPI3-EDTA Conjugates: A New Class of DNA Cleaving Agents” J. Org. Chem. 1993, 58, 3018-3024.
