T.J. Campbell and Dr. Barry Bickmore, Geology
The struggle to find and maintain sources of clean water faces billions of people throughout the world. Increases in groundwater contamination have made this problem even more severe. It is therefore of great importance to understand how contaminants travel and interact in the subsurface. Chemical reactions with the surrounding rock often control the movement of these contaminants. Mineral surfaces can become charged, attracting oppositely charged contaminant particles and slowing their progress. This surface charging is dependent on the reactivity of the individual functional groups on mineral surfaces. Reliable predictions of the reactivity of these groups are necessary to improve the tracking and remediation of contamination.
The multisite complexation (MUSIC) model attempts to predict the reactivity of these surface functional groups (Hiemstra et al. 1996). The simplest predictions made by this model deal with acid-base reactions. These reactions can control the charge of a mineral surface. However, it has been very difficult to experimentally test these predictions because mineral crystals are usually composed of distinct crystallographic surfaces, which have different types and amounts of functional groups. Traditional wet-chemical experiments can measure the reactivity of the entire surface, but not of individual faces or groups. Recent advances in atomic force microscopy (AFM) have allowed us to measure relative changes in the surface charge of specific crystal faces of minerals, but the behavior of different types of surface functional groups on these surfaces cannot always be separated.
The mineral gibbsite (Al(OH)3), however, presents an interesting opportunity. Its basal (001) face is populated entirely by one surface functional group, (>Al2OH). Therefore, the charging on this face can be attributed to the acid-base behavior of this one group. Based on the estimated acidity constant of this group, the MUSIC model predicts this face to be proton unreactive over a pH range of 4-10. Bickmore et al. (2002) have calculated a different acidity constant, taking into account surface relaxation. This would predict the face to be proton reactive over this same pH range.
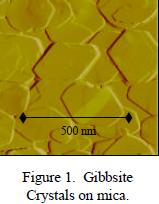
The first objective of this project was to synthesize gibbsite crystals and fix them to a flat surface that could be used as a stage under the AFM. We created a procedure to do this by altering a published methodology. After a number of failed attempts, a working procedure was found. The crystals, which averaged around 300 nm in length, were created from a titration of 4M NaOH and 1M Al(NO3)3. Mica flakes were inserted into the Al(OH)3 precipitate to act as a flat stage. The precipitate was then cooked at 40ºC for several weeks to allow the crystals to grow on the mica surface.
The Mica flakes were then placed in the fluid cell of the AFM and examined. The images revealed that the mica had been coated with hexagonal crystals. This confirmed that the Al(OH)3 precipitate had properly crystallized to Gibbsite.
The second objective of the research was to use the AFM to qualitatively measure the surface charging of gibbsite crystals. Relative changes in surface charging can be inferred by measuring changes in the adhesive (or repulsive) forces between an AFM tip and the sample surface (Fig. 1) over a pH range. The interactions involved include electrostatic, Van der Waals, and hydrogen bonding forces. Only the electrostatic and hydrogen bonding forces vary significantly with pH, and the electrostatic forces are typically much stronger. Therefore, changes in the adhesive force can be interpreted to mimic changes in electrostatic charging.
This adhesive force, called the pull-off force, was measured on various samples over a pH range of 3-10. Imaging mode would be used to locate a flat crystal, then the pull off force would be measured, then a new pH would be injected into the fluid cell. This procedure, however, turned out to be more difficult than expected. Because of the sensitivity of the AFM, injecting new solutions would often disrupt the tip causing a loss of signal. Many different procedures were implemented to reduce the likelihood of this disruption; however, this remained a difficult aspect of the experiment. After approximately 50 experiments only three full and correct data sets were acquired.
The last objective of the research is to analyze and interpret the data. If the Gibbsite surface is proton reactive there should be a transition in charging behavior around a pH of 5.2 as the aluminol groups reach their pKa. This should be represented by a change in inflection of the adhesion graph. This general trend is seen in the graphs; however, further analysis is needed. These graphs currently represent the sum of surface charging on both the silicon nitride tip and the Gibbsite surface. Even though, silicon charging is fairly even, further work will be needed to separate this behavior from that of Gibbsite surface charging. Currently data on the charging behavior of silicon is being acquired and a procedure to subtract this data from our data is being created.
These initial results will continue to be analyzed and eventually be incorporated into a paper to be submitted for publication.
References
- Bickmore, B.R., Rosso, K.M., Nagy, K.L., Cygan, R.T., 2002, Ab Initio Determination of dioctahedral 2:1 phyllosilicate edge surface structures: implications for acid-base reactivity: in review Clays and Clay Minerals.
- Hiemstra, T., Venema, P., Van Riemsdijk, W.H., 1996, Intrinsic proton affintity of reactive surface groups of metal (hydr)oxides: the bond valence principle: Journal of Colloid and Interface Science, v 184, p. 680-692.
