Trever Bradley Burgon and Dr. Gregory F. Burton, Chemistry and Biochemistry
Introduction
Human Immunodeficiency Virus (HIV), the retrovirus that causes Acquired Immunodeficiency Syndrome (AIDS), currently infects over 42 million people; more than three million of them are children under the age of 15. The Joint United Nations Program on HIV/AIDS calls the current AIDS epidemic: “the most devastating disease mankind has ever faced.”
There is no cure for AIDS. When first introduced in the early 1990’s, HAART (highly active antiretroviral therapy) offered the first hope that a cure for AIDS was near. HAART limits the ability of HIV to replicate (1) but was later found to be unable to completely block replication or remove HIV from the body. When patients stop taking HAART, their viral load and symptoms quickly return to pretreatment levels because virus remains in reservoirs in a persistent and nonreplicating state, untouched by HAART. After treatment, these reservoirs release infectious virus to reignite infection. Follicular dendritic cells (FDCs), located in the germinal centers of secondary lymphoid tissue, have previously been shown to trap and preserve highly infectious HIV on their extracellular surface (2). This unique interaction between FDCs and HIV led us to hypothesize that FDCs are a reservoir that traps and preserves HIV for extended periods of time in infected humans and may contribute rebound virus upon treatment termination.
In the body, the population of virus is constantly evolving as it adapts to changes in a patient’s immune response and drug regimen. This viral evolution creates many quasi-species, genetically similar yet distinguishable virions. At any given point in infection, the quasi-species that are most able to infect and replicate under the existing conditions will be present in productively infected cells, usually T-cells. As conditions change over time, new quasi-species that are better suited to the new conditions will outgrow and replace the previously successful quasi-species.
We hypothesize that FDCs trap extant viral quasi-species and then preserve them after they have been lost from the productively infected cells. Two predictions stem from this hypothesis: (1) Because the FDC-trapped HIV population should include quasi-species trapped at many time points during infection, it should be more genetically diverse than virus found in productively infected cells, and (2) Some HIV quasi-species trapped on the FDC should be phylogenetically similar to quasi-species isolated from productively infected cells isolated earlier in infection. To test our hypothesis we cloned, sequenced, and phylogenetically analyzed the HIV polymerase (pol) gene, isolated from FDCs and T-cells in an infected patient at autopsy. To better understand the role of FDCs as HIV reservoirs we sought to characterize both the diversity of the FDCtrapped HIV population and the similarity of FDC-trapped HIV to virus isolated from productively infected cells at 4 and 18 months before death.
Materials and Methods
HIV DNA was isolated from coculture assays and amplified by nested-set PCR. Pol amplicons were ligated into a plasmid vector cloned into bacteria. Plasmid DNA was then sequenced. Sequences were read at the BYU core sequencing facility and edited using SEQUENCER. Nucleotide alignments were done with CLUSTAL X and edited in MacClade. The best-fit evolutionary model was selected in MODELTEST version and determined to be HKY+I+G. This model was then used in PAUP* to construct a maximum likelihood tree.
Results and Discussion
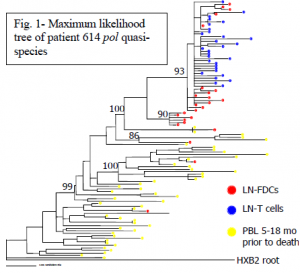
The phylogenetic analysis is represented in Figure 1. Genetic differences are represented on the tree by the horizontal distance separating the different quasi-species. The greater the horizontal distance, the greater the difference. HIV isolated from T-cells (blue dots) formed a relatively tight cluster at the top of the tree with little relative diversity within the population. FDC-isolated quasi-species (red dots), however, were spread throughout the tree. There is more genetic diversity within this population, consistent with our prediction that FDC-trapped HIV would be more genetically diverse than the extant HIV population represented in the T-cells. These data support our hypothesis that FDCs are a reservoir of HIV that trap and preserve quasi-species throughout infection.
During infection blood samples were drawn from the patient at two time points, and HIV pol isolated from peripheral blood lymphocytes (PBLs) was cloned and sequenced (yellow dots). None of the T-cell quasi-species cluster with the virus present earlier in infection, suggesting that the previously successful quasi-species are no longer represented in the productively infected pool. Four FDC-isolated quasi-species, however, do cluster with the blood samples that were taken months before autopsy. This clustering is consistent with our hypothesis that some FDCassociated virus has been archived from earlier time points in infection. The most recent blood sample was from October 2001 (four months before the patient died). This clustering of FDCtrapped virus with quasi-species that were in the productively infected pool four months previous gives us a conservative estimate for a time point of trapping. The phylogenetic data suggest that the FDC-trapped virus that clusters with PBL-isolated virus has been preserved in an infectious form for at least four months. These data indicate that FDCs are reservoirs of infectious HIV in humans that may contribute to post-treatment rebound (3).
References
- Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The Challenge of Viral Reservoirs in HIV-1 Infection. Annu. Rev. Medicine. 53:557-593.
- Burton, G. F., Keele, B. F., Estes, Jacob D., Thacker, Tyler C., and Gartner, S. 2002. Follicular dendritic cell contributions to HIV pathogenesis. Seminars in Immunology. 14: 275-284.
- Acknowledgements- Brandon Keele, Benjamin Farnsworth
