Lael J. Stander and Dr. Scott C. Steffensen, Psychology and Neuroscience
Intra-cranial self stimulation (ICSS) has been well documented in both animal models and humans. Stimulation of various centers in the brain creates a very powerful reinforcement. Subjects will prefer ICSS to food, mating behavior, and drugs of abuse. One such pleasure center is the medial forebrain bundle (MFB) which functions as an information highway between several other pleasure mediating structures in the limbic system. Interestingly, constant stimulation of the MFB produces a highly dysphoric response. Animals respond with aversive behavior similar to pain avoidance. What is the electrophysiological change that mediates this reversal of pleasure to pain? In order to address this question, we established three criteria for analysis: Behavioral activity, electroencephalographic data (EEG), and single cell recordings in the Ventral Tegmental Area (VTA), a brain region previously implicated in addiction and reinforcement.1
Sprague-Dawley rats were fitted with an intracranial stimulator in the MFB and EEG recording electrodes in the frontal subarachnoid space. After recovery they were placed in an operant chamber with two photo-electric triggers. They were trained with free access to brief (500ms) stimulation of the MFB which they aggressively pursued by interrupting the photo-beam with their nose. Once robust behavior had stabilized, a left nosepoke triggered a constant stimulus to the MFB while the right trigger terminated the stimulus. During this training period, the rats began to show aversive behavior after approximately four seconds of stimulus. They where shaped during training to turn off the stimulus. All rats studied chose to terminate the stimulus after a duration of between 2.5 and 3 seconds. The interim between stimulations was far more variable, ranging from an immediate return to stimulation to a break of several minutes.
We continuously recorded the EEG data from the rats during ICSS. The EEG data gathered during stimulation was corrupted by artifact from the electrode, so that period was ignored. Both spectral analysis and time-locked amplitude studies revealed no significant EEG activity related to the self administration of ICSS.
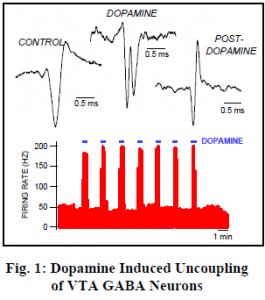
In order to record directly from neurons, anesthetized rats were placed in a stereotaxic device and a glass micropipette was inserted into the VTA. Stimulating electrodes were inserted at various locations known to induce ICSS behavior. One of our most striking discoveries was termed Dopamine Induced Uncoupling of VTA GABA Neurons (neurons that use ã-amino butyric acid as their transmitter).
We filled our recording pipette with Dopamine, a powerful neurotransmitter which is highly excitatory to VTA GABA neurons. This drug can then be injected by iontophoresis into the fluid surrounding the very neurons from which we record. We found that what appeared to be a single neuron actually split into two separate action potentials when we administered dopamine. This implies that the two spikes are coupled by electrical synapses ten times faster than any yet discovered!
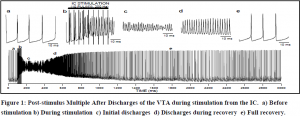
We had previously found that stimulating the internal capsule (IC) causes a massive activation of the VTA called post-stimulus multiple after discharges (PSDs). We decided to study these in coordination with gap junction blockers to see if the discharges would be affected. Several of these agents inhibited PSDs including Quinidine which is a specific blocker of connexin 36. This supports the hypothesis that these discharges are mediated through gap junctions. Ethanol also inhibited PSDs, but GABA modifiers such as chlorodiazepoxide, brevital and heroin had little effect on PSDs although they profoundly modulated spontaneous firing rate. We also performed intracellular recordings of PSDs in unanesthetized rats. Figure two represents one such recording (10 pulses to the IC at 200 Hz, 2.0 mA). The oscillations during PSDs (part c,d) did not ever drop below threshold, so the voltage gated Na channels never had an opportunity to reset. It seems then, that PSDs may represent not an action potential, but a series of gap junctions oscillating in a feed-back circuit.
This data refutes the wide belief that electrical synapses are very rare or even absent in higher mammalian species, demonstrating electrical synapse conduction along an extensive area of the midbrain in rats. This “neurological superconductor” is a prime target for future substance abuse research because it represents circuitry capable of modulating the entire limbic system at unprecedented speeds and is capable of tremendous signal amplification.
As frequently happens with primary research, the most significant discovery made through this project does not answer the original question posed. The behavioral data seem to imply a reversal of perceived pleasure to pain between 2.5-3 seconds after stimulation of the MFB. EEG data was ambiguous and provided no significant information. Single-cell recording of VTA neurons showed that there is likely a large network of limbic neurons interconnected with gap-junctions.