Jarom D. Webster and Dr. Thomas H. Fletcher, Chemical Engineering
Introduction
The black liquor recovery boiler is the largest and most expensive piece of equipment in a modern pulp mill. Inside this boiler, black liquor is burned to produce electricity for the mill and also to recover spent chemicals. Black liquor combustion occurs in three stages: drying, pyrolysis, and char combustion. Understanding black liquor pyrolysis is an important step to controlling and improving the efficiency of the recovery boiler.
Specific Objectives
The purpose for this experiment was to provide information concerning the organic mass loss that takes place during black liquor pyrolysis, both in a flat-flame reactor and a high-temperature furnace, at varying temperatures and residence times. The results of these experiments will be used to evaluate computer models of the black liquor combustion process.
Experiment Description
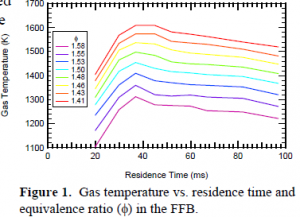
The black liquor pyrolysis experiments were conducted in a reactor system consisting of a Hencken flat-flame burner (FFB) and a 30-cm-high quartz tower used to confine the postflame gases. Flow rates of CO, N2, H2, and air were adjusted to obtain a flat, non-sooting flame. Carbon monoxide was used as a fuel to maintain a low-temperature, stable flame in fuel-rich conditions1. The flame temperature was adjusted by varying the equivalence ratio (f). Figure 1 shows how the gas temperature varied with varying equivalence ratio and residence time.
A syringe-type particle feeder was used to provide a steady-state flow rate (~0.5 gm/hr). The black liquor particles were entrained in N2 and were injected through a centerline metal tube, the end of which was about 1 mm below the CO flame. The temperature in the FFB was adjusted by changing equivalence ratio. The reactor was situated on a movable platform, which could be raised or lowered in relation to the sampling probe to achieve different residence times. A watercooled probe with nitrogen quench jets at its tip collected all reaction products. These reaction products were then separated, measured and stored2.
Results and Discussion
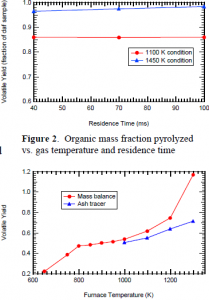
Experiments in the FFB were conducted at two temperature conditions: 1100 K and 1450 K. The Kraft black liquor, obtained from Georgia, was dried and sieved to 45-75 ìm in diameter before being mixed with silica gel beads (to prevent clumping inside the feed tube). Equivalence ratio was varied to change the particle temperature. The experiments were carried out in random order, with three replicates run at each temperature (1100, 1450 K) and distance (1, 3, 5 in.). After each run, the char sample collected in the cyclone was weighed and stored in argon to await further analysis. The soot/tar was collected from the polycarbonate filters, weighed, and stored in a similar fashion. Figure 2 shows the organic mass fraction pyrolyzed as a function of particle temperature and residence time.
Separate sets of pyrolysis experiments were performed in a furnace (Thermolyne Corp.) at temperatures ranging from 650 to 1300 K. Each run was purged with N2 during the entire holding time, which was ten minutes in all cases. As expected, the mass loss increased as the temperature of the furnace was raised. The black liquor experienced more swelling at higher temperatures. It was anticipated that some of the sodium in the ash would react at the higher temperatures (1000-1300 K). Consequently, an ash tracer technique was used to give an organic mass loss (daf basis) at each temperature. Additional analysis of the ash is planned using the inductive coupled plasma (ICP) technique, with Ti or Si as a tracer.
Figure 3 shows the results of the furnace experiments. As expected, the organic mass fraction pyrolyzed increased as the ambient temperature was increased. However, the mass balance showed volatile yields that were greater than 100% at high temperatures. Volatile yields of 50 to 70% daf were determined at these same temperatures by measuring the mass and the amount of ash before and after reaction. This indicates that some of the mineral matter in the black liquor sample vaporized at temperatures above 1000 K.
Conclusion and Acknowledgements
Quantitative experiments have shown that the extent of organic mass loss during black liquor pyrolysis depends both on particle temperature during reaction and residence time. Future experiments will focus on the pyrolysis of several different types of black liquor particles, along with the devolatilization of black liquor droplets.
I give special thanks to Dr. Tom Fletcher for his help, support and encouragement.