Steven Gale Smith and Dr. Calvin Bartholomew, Chemical Engineering
Catalysts are widely used throughout many industries to decrease reaction time and make processes economically favorable. In the synthetic fuels industry a catalyst is used to convert carbon monoxide gases (CO) to hydrocarbon liquids (CnH2n+2). In this process, the catalyst becomes coated with the heavier hydrocarbon products. This coating is a wax and deactivates the catalyst (inhibits the catalyst from reacting). The catalyst is currently one of the largest expenses for this process and prevents the process from being competitive with natural fuels. Hence, a method to remove the waxes from the catalysts was studied. The process of removing the wax from the catalyst is called regeneration. Two methods of regenerating the catalyst were compared in this experiment, Soxhlet Extraction and Supercritical Fluid Extraction (SFE).
Experiments were performed using an alumina sample and wax created at BYU during FT tests of a cobalt catalyst. The alumina sample was impregnated with wax to simulate a deactivated catalyst by adding a 1:1 ratio (by weight) of both alumina and wax samples, then heating in solution with approximately 100 ml of heptane and boiling until most of the heptane had boiled off. The solution was then left overnight and dried the following day in a drier for 3 hours each at 50°C then 100°C. Drying above the boiling point (98°C) assured that negligible amounts of heptane remained in the sample. This sample was then tested to determine the approximate effectiveness of both the Soxhlet and SFE experiments.
It was intended that the sample would be a 50/50 mixture of alumina and wax but in the course of the experiment it was found that some of the wax was lost. Accordingly the fraction of wax (%f) in the sample to be extracted was difficult to quantify because mass was lost during preparation due to (1) bubbling and splashing (which would cause both alumina and wax to be lost) and (2) volatiles in the wax being released to the atmosphere. It was found that if all the mass loss were assumed to be due to volatilization, the sample after preparation would be 18% wax by mass. This was found to be unrealistic because calculated extraction percentages using Equation 1 exceeded 100%. It was concluded by estimating the losses during preparation that the most reasonable value of %f would be 30% ± 5% wax by mass.
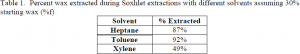
Soxhlet Extractions The Soxhlet apparatus consists of three parts, bottom, middle, and top. The bottom is a bulb where the solvent is loaded and boiled. This bulb is placed in a heated bowl. The middle section is where the deactivated catalyst sample is loaded inside a thimble. In the top section, cold water flows around the outside causing condensation. In the Soxhlet extraction solvent vapor travels up the column to the top where it condenses and drips down onto the catalyst wax mixture. When the solvent in the middle section reaches an overflow level, it is recirculated to the solvent bowl and reboiled. The Soxhlet extractions were conducted using this procedure with three different solvents (heptane, toluene, and xylene) for a period of 24 hours. The mass of the sample was weighed before and after the extraction, the change in mass being the amount of wax that was extracted. The percentage of the wax extracted for each of these solvents relative to the total wax on the catalyst can be seen in Table 1.
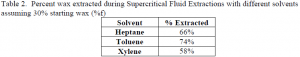
Supercritical Fluid Extractions The Supercritical Fluid Extractions were done using carbon dioxide and another solvent (heptane, toluene, or xylene) in an extraction cell under high pressure and temperature. The extraction cell was packed with the wax/catalyst mix and with glass wool. The solvent was then added to fill the cell. The cell was pressurized to 200 atm using supercritical grade carbon dioxide and heated to 150°C using an aluminum heating block. The cell was maintained at this pressure and temperature for two hours. After two hours, a valve was opened that released the carbon dioxide, solvent, and wax into a collection vessel. Once the cell reached atmospheric pressure, it was pressurized again and maintained for another two hours. This was done so that there were three two-hour periods for each extraction. After the last period, the extraction cell was opened up and the contents removed and weighed. In some cases, traces of the solvent remained on the sample. Therefore, the sample was given time to dry or was dried in an oven. A mass balance was done to determine the weight of the wax that was extracted. The results for the different solvents are shown in Table 2.
In comparing the two methods, the Soxhlet method appears to be approximately 20% more effective in extracting the wax from the alumina with heptane and toluene as the solvents but the SFE is about 10% more effective using xylene. Toluene was the most effective solvent in both the Soxhlet and SFE methods. This experiment has shown that using a Soxhlet extraction method with toluene as the solvent should be the most effective method to extract waxes from deactivated catalysts.