Kelly Jeppesen and Professor Travis Oliphant, Electrical Engineering
Light scattering is a means of detecting certain chemical properties of a sample of material. As a photon (light packet) encounters any material, there is some probability that the photon will be absorbed. The energy from an absorbed photon will either work to heat the sample or be reemitted. When re-emitted, a photon will not necessarily travel in the same direction or with the same phase in which it encountered the material. Also, the amount of energy re-emitted via scattering is dependent on the incident light wavelength and properties of the material, which, for example, is why the sky looks blue. Light scattering of materials can be easily measured by placing detectors at angles from a single light source. For the sake of this research project, light scattering at 1300 nanometers and 1550 nanometers (common optic fiber transmission frequencies) will be measured for various biological substances, e.g. proteins and DNA.
It is hoped that results of the experiment will shed some light on how proteins operate. Proteins have many different biological functions, and it is known that for any given protein, a certain amount of bound water is needed in order to endow the protein with proper functionality. It has also been conjectured that some of proteins’ function is to move surrounding water particles in such a way that it sends a signal downstream to other proteins.1 Since water bound to a protein behaves differently than water in bulk, by measuring wavelength differences in the incident and scattered light off a protein, we may be able to better understand how tightly the water is bound.
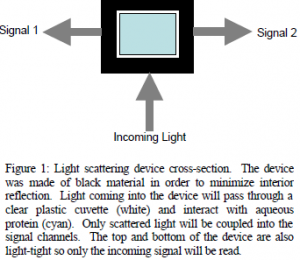
Preliminarily, study needed to be conducted concerning the setup to be used in the light scattering device. Dr. Ken Solen of the Chemical Engineering Department, who has done some significant work with light scattering experiments, was helpful in walking us through the process of configuring the system and providing applicable literature. For the purposes of our project, we had to come up with an original design for the light chamber. A simple diagram of the device is given in figure 1
After obtaining specifications for the machining of an SMA fiber optic port (the connection chosen for the device because of its simplicity), plans were sent to the campus’s precision machine lab for fabrication. While the device was being machined, further research was conducted concerning the measurement devices. Our department owns an Agilent Optical Spectrum Analyzer capable of detecting wavelength differences on the order of .1 nanometers.
Unfortunately, for the purposes of this experiment, that sort of resolution won’t tell us much. However, higher resolution can be achieved by placing the device in an interferometer, which instead of measuring the signal from the protein directly, measures it against the incoming signal.
light-tight so only the incoming signal will be read. Another problem encountered is that the optics lab on campus doesn’t have an SMA-FCPC converter. FCPC cable endings are the most widely used in the lab and are required for connection with the laser and oscilloscope to be used. In order to overcome this, we will soon be splicing some of our own cables in order to create the connections needed.
We anticipate taking some preliminary measurements with the device this month. After measuring the possible biases of the light chamber, we will take some control measurements with water samples and begin looking at various concentrations of some common proteins. These preliminary measurements will be taken and documented by the end of the school year.
References
- See Pollack, Gerald H. Cells, gels and the engines of life : a new, unifying approach to cell function, Ebner & Sons, Seattle, WA (2001)