Tiffany Sabin and Dr. Barry Willardson, Chemistry and Biochemistry
Cellular signaling is vital to the proper functioning of a cell. In the visual system, as light activates the transmembrane receptor rhodopsin, a series of signals is initiated throughout the cell. This response begins with the activation of G proteins which couple to the rhodopsin. One important player in G protein signaling is Phosducin (Pdc). The unphosphorylated form of Pdc binds to the βγ subunit of the G protein.1 We used a proteomics approach to investigate the possibility of other binding partners for Pdc.
A recent method for identifying binding partners of proteins has been developed. Tandem Affinity Purification (TAP) utilizes a system of affinity tags which can be used under mild conditions.2 The TAP tag involves a calmodulin-binding peptide, a TEV protease cleavage site and protein A. We prepared a cDNA construct of Pdc in the E. coli expression vector pET 15b. This construct includes an N-terminal hexahistine tag and a C-terminal TAP tag. We grew the tagged Pdc in E. Coli and purified it using the hexahistine tag.
After the tagged Pdc was obtained, we prepared light and dark adapted retinal extracts from bovine retina. Each extract was divided in two and these portions were incubated with phosphorylated or unphosphorylated Pdc. TAP columns were run on these extracts, and the eluent was analyzed by gel electrophoresis and Sypro Ruby staining. In eluent from all four columns we found two co-eluting bands besides the one corresponding to Gβ (see Figure 1). A mass spectrometric approach was used to identify these bands.
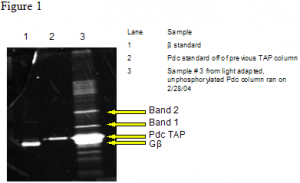
Bands 1 and 2 were cut out, in gel digested with trypsin, and analyzed on a Q-Star/Pulsar quadrupole time-of-flight mass spectrometer in MALDI mode. This data was compared with theoretical mass fingerprints of the NCBI protein database using the Mascot application (Matrix Science). Band 1 was identified as a bacterial elongation factor, merely a contaminant from the E. Coli in which the Pdc was expressed. Band 2 was identified as alpha tubulin, a cytoskeletal protein. To determine the significance of the alpha tubulin interaction, binding experiments will be performed to determine the affinity of Pdc for alpha tubulin.
References
- Arshavsky, V.Y., Lamb, T.D. and Pugh, E.N. Jr. (2002) Annu. Rev. Physiol. 64, 153-187.
- Rigaut, G., Schevchenko, A., Rutz, B., Wilm, M., Mann, M. and Seraphin, B. (1999) Nature Biotechnology 17, 1030-1032.
