Jeffrey Sparenborg and Dr. Scott Steffensen, Psychology Department
I set out to discover the substrates of the brain that are involved with chronic use of alcohol. My mentor Scott Steffensen had previously found that the GABA neurons in the midbrain, more specifically, in the ventral tegmental area (VTA) of the midbrain were susceptible to ethanol administration. This information is only a piece, however, of the puzzle that will eventually show the full picture of how ethanol disrupts our brain’s function. The next piece that my ORCA aimed to delineate was to see what mechanisms in the VTA were changing when the brain became addicted to alcohol i.e., what physiological component is responsible for the tolerance or withdrawal effects of a long-term drinker.
The experience was an amazing opportunity for hands-on application of what I had been learning in my undergraduate neuroscience courses. We were using electrophysiological technique to record the firing rate of neurons in the VTA. What this means is that we would make a very small glass pipette, which acted as a recording electrode, and place this in the VTA of an anaesthetized rat. The normal activity of the GABA neurons were compared with the firing rate under administration of ethanol, and other drugs that modulated specific receptors e.g., NMDA and GABA receptors and Gap Junctions.
In order to see what was changing under chronic ethanol conditions we would need to record how a specific neuron was firing before any ethanol exposure and then record the same neuron weeks later following long-term exposure. This task proved more difficult than anticipated. We first took an electrode and placed it on a GABA neuron in the VTA. This recording electrode would have to be stabilized in its exact place as a cranial implant so that we could collect data from it throughout the course of the experiment. Many rats were undergoing this procedure, but the electrodes weren’t picking up the neuron activity when we would try to record from the same rat, days later. After numerous adjustments in electrode components and surgical procedures, we had to abandon the technique and shift our focus to study ethanol’s acute influence on the VTA GABA neurons.
This change in focus was by no means a loss to our work, just a change in directions that the lab needed to take. We were still carrying out the same protocol (as described above) to see the physiological adaptations of these neurons; however, now we were collecting all of the salient data in one day rather than over the period of a month.
This figure shows how the discharges in the VTA come to an arresting halt under treatment of ethanol. The comparison of saline is shown as a control to show the normal discharge rate.
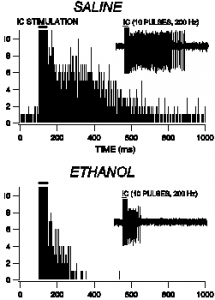
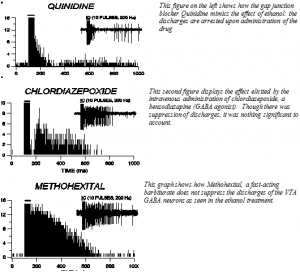
From what our data show, both the ethanol and gap junction blocker (quinidine) act through suppression of VTA GABA neuron discharge activity. Interestingly, the GABA modulating sedative Hypnotics such as Chlorodiazepoxide and Methohexital do not involve themselves with the discharges of VTA GABA neurons. This project has helped to outline one more critical piece to the puzzle of how ethanol acts on the central nervous system.
