Brian C. Bingham and Dr. Scott Steffensen, Psychology
Alcohol addiction has destroyed lives and communities for centuries. By understanding the mechanism of how our brain becomes addicted to alcohol we can better solve the problems this addiction creates. The current theory states that dopaminergic neurons in the ventral tegmental area (VTA) of the midbrain help produce feelings of reward associated with alcohol use. These dopamine neurons are modulated by GABA neurons that form a feedback loop that can be stimulated via a brain structure called the internal capsule. The purpose of this project was to clarify the effect of dopamine (DA) on GABA neuron firing rate and electrical coupling.
Methods
To characterize the effect of DA on GABA neurons we used an electrophysiological method called microelectrophoresis. In this method we dissolve the dopamine in a solution with KCl and fill glass recording pipettes with this solution. These pipettes have tips that are broken to about 2-3µm. The electrodes are connected to an oscilloscope and to a computer that allows us to locate individual neurons based on their electrical activity and to characterize their wave patterns and firing rates. Because dopamine is a charged molecule in solution we are able to hold the dopamine in the pipette by applying a small holding current of -10 nA. Once we find a neuron that we can recognize as a GABA neuron in the VTA, based on its waveform and the location of the pipette with respect to the rat’s skull, we can release dopamine from the pipette by changing the holding current from -10nA to +20nA. This in effect expels the positively charged dopamine from our pipette into the area surrounding our neuron of interest. We then can compare the waveform and firing rate of the neuron before DA application and after.
When we began the study, we knew that DA increased the firing rate of GABA neurons, but we didn’t know the mechanism involved. Also, we had noticed that upon application of DA the waveform of the GABA neuron would change from one spike to two spikes. It has been shown that gap junctions interconnect VTA GABA neurons so we assumed that this apparent “splitting” was due to an interruption in this gap junction which caused a delay in the firing of two adjacent neurons. To test these two observations, we took controls with and without microelectrophoretic application of dopamine and then we gave quinidine, a gap junction blocker, via a jugular catheter to assess whether dopamine induced firing rate increases and electrical “splitting” were modulated by gap junctions.
Results
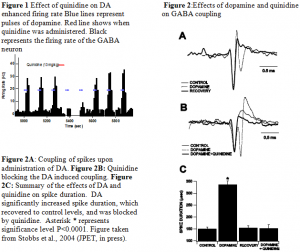
Firing rate: Microelectrophoretic application of dopamine markedly increased VTA GABA neuron firing rate 131% (mean baseline firing rate = 26 ± 3.1 Hz; mean dopamine firing rate = 60 ± 4.5 Hz). Figure 1 shows the effect of quinidine on the firing rates of GABA neurons when given systemically with in situ pulses of dopamine. As shown, quinidine destroys any firing rate enhancement by DA for a short period of time.
Spike Duration: Concomitant with the enhancement of firing rate, microelectrophoretic application of dopamine enhanced VTA GABA neuron spike duration 124% (Figure 2). The widening of the spike by dopamine would often result in two distinct spikes on the waveform. These trailing spikes were often smaller spikes that comprised one, or sometimes two, of the background spikes in a cluster of phasic, mildly synchronized neurons. Systemic application of quinidine (10 mg/kg iv) reversibly blocked dopamine enhancement of VTA GABA neuron spike duration and recruitment of trailing spikes. The leading spike waveforms are unchanged by dopamine suggesting that the waveform results from coupling spikes not splitting spikes as we had previously thought.
Discussion
The ability of quinidine to block the dopamine enhancement of GABA firing rates and the recruitment of background spikes provides support to the hypothesis that VTA GABA neurons are connected through electrical synapses and that part of their dopamine pharmacology is mediated through these synapses. One of the surprising results of this study is that application of dopamine actually recruits other GABA neurons instead of splitting the connection between them. This makes sense however, when considered with the increased firing rate upon dopamine application. The next step in this research is find out which second messenger systems are involved from the dopamine receptor to the gap junctions and to find out if alcohol is operating on these second messenger systems to yield an effect similar to that of quinidine.