Taylor Turner and Dr. William Pitt, Chemical Engineering
Previously it has been shown that antibiotic killing of bacteria, especially biofilms, can be enhanced by various non-invasive treatments. Low-level electric current has been shown to increase killing of Streptococcus gordonii biofilms when applied during treatment with gentamicin.1 Others have partially tested the feasibility of electrically killing bacteria on prosthetic joints.2 Ultrasonic exposure has also been shown to increase killing of bacterial biofilms.3 Our group previously observed that ultrasound increased the transport of gentamicin through biofilms.4 Electrical treatment of S. gordonii in the absence of gentamicin also generates a 1.9 log reduction in biofilm cell counts.1 Our previous findings showed bacteria diluted in different solutions showed different levels of killing. My goal was to characterize and explain this electrical killing.
Agar solutions were melted until completely liquid then cooled to approximately 40º C before adding bacteria. The bacterial suspension was then placed in custom apparatus designed for applying electrical current to liquids or gels, respectively. Overnight culture diluted in PBS was pipetted into the sterile liquid apparatus (see Fig.1); or culture suspension diluted into ½ concentration agar was pipetted onto the sterile flat plate apparatus (see Fig. 2) and allowed to cool. Electrical current was applied to the suspensions, and samples were removed at 0, 30, 60, and 90 minutes. Samples were removed from the vertical tube apparatus through a small hole in the upper plate and serially diluted into PBS. Trials run in the flat plate apparatus used a separate apparatus for each time period and samples were removed by cutting a 62.6 µL disc then serially diluted in PBS. The agar discs were vortexed until broken up in the original PBS dilution before additional dilutions were made.
![]()
The respective dilutions were plated on nutrient agar plates. Five 10 µl drops were plated for each dilution and the plates were then incubated overnight at 37º C. The following day, the colonies were counted manually in accordance with published drop plate methods.5
Five distinct situations were tested: Bacteria suspended in ½ concentration agar @ 150 µA/cm2, bacteria suspended in ½ concentration agar @ 300 µA/cm2, bacteria suspended in ⅛ concentration @ 300 µA/cm2 in vertical tube apparatus, bacteria suspended in ⅛ concentration @ 300 µA/cm2 in flat plate apparatus, and bacteria diluted in PBS @ 300 µA/cm2. Currents were kept constant throughout the 90 minute periods. All trials were performed at room temperature.
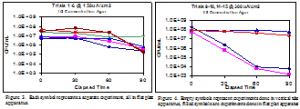
Bacterial killing was shown to increase with the application of electrical current. The graphical summaries of the results can been seen in figures 3-6. In trials at 150 µA/cm2 bacterial counts decreased by 1-2 orders of magnitude. When suspended in identical solutions and treated at 300 µA/cm2 bacterial counts decreased by 3-4 orders of magnitude. These results were consistent with expectations. Trial performed in ⅛ concentration agar were inconsistent, depending on which apparatus was used. Figure 4 show significant killing in the two trials performed in the flat plate apparatus, but trials performed in the vertical tube apparatus showed no such killing. Trials performed in the vertical tube apparatus in PBS also showed no significant killing. This is consistent with previous data (unpublished data).
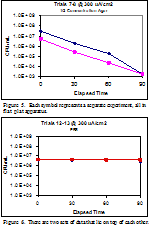
We observed that at higher current levels the bacteria died when immobilized in the agar suspension. This observation is consistent with the null effects that electrical current has on bacteria suspended in PBS. Agar suspensions of increasingly lower concentrations were made until one was found (approximately ⅛ dilution) that failed to form a solid at room temperature. Initial results on flat plate showed that ⅛ concentration killed similarly to ½ concentration (see Fig. 4), but continued testing in the vertical tube of the ⅛ concentration agar provided conflicting data. We found that even at the same current density, the killing of bacteria in the suspension was dependent on the apparatus geometry.
These results are inconclusive as to finding the cellular cause of bacterial death as a result of applied electrical current, but do provide a starting point for additional research. Further investigation may prove useful to the medical field, if electrical current could be used effectively to eliminate bacteria. The consistent problem of bacterial infection of surgical implants provides an arena wherein such a technique, if characterized, could be applied.
References
- Stewart PS, Wattanakaroon, W. 2000. Electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin. Archives of Oral Biology. 45:197-171
- Trampuz A, et al. 2003. Molecular and Antibiofilm Approaches to Prosthetic Joint Infection. SECTION I SYMPOSIUM: Proceedings of the Musculoskeletal Infection Society 2002. 1:69-88
- Williams RG, Pitt WG. 1997. In vitro response of Escherichia coli to antibiotics and ultrasound at various insonation intensities. Journal of Biomaterials Applications. 12 (1): 20-30
- Peterson RV, Pitt WG. 2000. The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli. Colloids and Surfaces B-Biointerfaces. 17 (4): 219-227
- Herigstad B, Hamilton M, Heersink J. 2001. How to optimize the drop plate method for enumerating bacteria. Journal of Microbiological Methods. 44:121-129
