Jeffrey Watkins and Dr. Branton J. Campbell, Physics and Astronomy
Layered transition-metal-doped bismuth vanadates, commonly known as BIMEVOX oxides, have very attractive oxygen-ion conductivity properties at moderate temperatures and show promise in applications as electrode materials and membranes for oxygen separation. Of great interest is the relationship between the atomic structure of BIMEVOX materials and their useful physical properties. We are investigating this relationship in order to better understand which structural features produce the best ionic conductors. This research requires the preparation of pure powder and single-crystal samples. Here, we present a novel sol-gel synthesis procedure that reduces preparation time, reduces impurities and yields superior single-crystal samples.
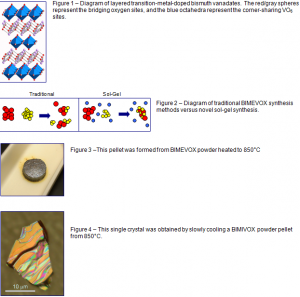
Amongst solid-state ionic conductors, the layered bismuth vanadates (Fig. 1) have the highest known oxygen conductivities. The bridging oxygen sites (red/gray) that connect the corner-sharing VO6 octahedra (blue) are actually 25% vacant. With shuffle-puzzle-like motion, oxygen atoms and vacancies flow in opposite directions within the vanadium-oxide sheets under the influence of an applied voltage.
The traditional BIMEVOX synthesis methods (Fig. 2) use high temperature annealing of the different components. This method relies on the mechanical and diffusive mixing of the individual atomic species. Clumps of material must be physically broken down with pressure and heat before a chemical reaction occurs. Traditional high-temperature syntheses typically involve slow annealings at 750°C, leading to Bi evaporation and the subsequent formation of impurity phases. Our sol-gel method, however, completely dissolves the precursors in an aqueous solution which allows and provides atomic-scale homogeneity at the outset (Fig. 2). The desired chemical reactions then proceed quickly to completion at much lower temperatures (570° C) and without mechanical grinding.
Single-crystal BIMEVOX samples (Fig. 4) are grown from pressed powder pellets (Fig. 3). The pellets are heated to the melting point of BIMEVOX (around 850°C) and cooled slowly to encourage crystal formation. Crystal sizes range from 1-500 microns in size.
The broad features in the diffuse-scattering pattern from BINiVOX encode information about nanoscale oxygen vacancy defect structures in the material that may ultimately determine its macroscopic oxygen-ion conductivity. We plan to further investigate these structures using state-of-the art x-ray scattering instruments here at BYU and at national synchrotron x-ray user facilities. Our new x-ray diffractometer is equipped with a rotating anode source, focusing x-ray optics, a 16-megapixel x-ray camera, two kappa-geometry goniometers, and both high and low-temperature capabilities. It has been optimized specifically for the demands of these difficult experiments.
The novel sol-gel synthesis of layered transition-metal-doped bismuth vanadates presented here offers several advantages over traditional synthesis procedures. Through the sol-gel method, greater atomic homogeneity is achieved, along with lower reaction temperatures and shorter annealing times. These advantages result in higher-purity BIMEVOX powder products. We plan on synthesizing a wide range of Fe and Ni doped BIMEVOX powder products ranging from x=0.0 to x=0.6. With this wide range of compositions, single crystals will be grown and analyzed using x-ray diffraction and diffuse scattering techniques. Our single-crystal and powder samples will be critical to the characterization of defect structure-property relationships in the BIMEVOX family of solid-state oxygen-ion conductors.