Roane Noel and Dr. David Belnap, Chemistry and Biochemistry
The Poliovirus image to the left is from a collection of three-dimensionally reconstructed images of virus particles found at the website: http://www.cgl.ucsf.edu/Research/virus/capsids/viruses.html October 10, 2005. This image is identical to the type of Poliovirus image I reconstructed with the help of mentor Dr. David Belnap. (Our Poliovirus reconstruction was not formatted for a personal computer.) What can be visualized with this model is the protein coat, or capsid, that surrounds the RNA core of the virus. The four different colors represent the four different proteins that make up the capsid. It was our theory that there was a stabilizing protein located underneath the capsid. This protein that we predicted to be a stabilizing element for the capsid we referred to as the “pocket factor.” We knew from previous observation that a certain destabilizing of the protein capsid occurs that enables the RNA within to travel from within the capsid to the host cell. Unless the protein capsid destabilized, the RNA would not be able to get out. Once the RNA is safely delivered to the host cell, it is translated to DNA and inserted into the host cell’s genome. As a part of the host cell genome, it encodes the synthesis of the viral proteins. Therefore, the invading virus’ RNA effectively pirates the host cell’s metabolic machinery and employs it for the production and spread of more virus particles. How the virus capsid destabilizes enough so as to allow the RNA to escape is unknown. Our theory was that there existed a “pocket factor” that when present helped to maintain capsid stability. When removed, adequate destabilization could occur. If this were so, and if the pocket factor could be made to remain within the capsid, the infectivity of the virus would be thwarted! True there exist vaccines that prevent Poliovirus from causing the problems it caused only 50 years ago, but Poliovirus has a pathogenesis (method of infecting host cells) in common with many other viruses for which we have no vaccines. Therefore Poliovirus is an ideal model to study and understand for antiviral therapy research.
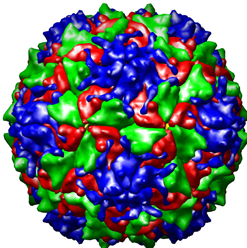
Creating a three-dimensional model was our answer to a microscopy problem. Viruses are so small that in order to obtain an image with reasonable detail requires the use of an electron microscope turned up to a very high magnification. Most viruses are similar to Poliovirus and as can be seen by the above image, viruses are relatively simple biological specimens. When subjected to intense beams of electrons in an electron microscope they become damaged. Therefore it is difficult to obtain high resolution pictures before the virus particles become destroyed. We took micrographs (micrographs are taken from an electron microscope just as photographs are taken from a light microscope) of nearly 9,000 poliovirus particles oriented randomly in ice. The micrographs were taken at a very low resolution so as to not damage and alter the physical properties of the particles. These images were collected and processed by the Mary-Lou super-computer on campus. The computer averaged the densities of the particles and was able to put together a three-dimensional model from the information.
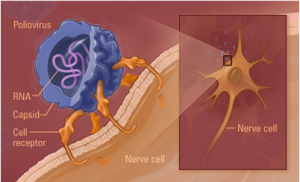
The model I made was one of poliovirus at the 160s stage. This is the stage that a free floating, inactivated poliovirus particle is in. Once destabilized the capsid changes conformation from a 160s to a 135s stage. It is the 135s stage that is the infectious stage. The 135s stage is illustrated in the image to the right. The illustration was provided by our collaborators in the James Hogle Laboratory at the Harvard School of Medicine and was retrieved October 10, 2005 at the following website: http://americanhistory.si.edu/polio/virusvaccine/how.htm. Our theory was that it is the removal of the aforementioned pocket factor that causes the 160s to 135s transition. To test this theory, we compared and contrasted the images of a 160s stage virion with that of a 135s stage virion. Dr. Belnap created the 135s stage model and I the 160s model. We performed a difference map between the two. A difference map notes the structural differences between two models. We hoped to find a difference in the pocket factor so as to find evidence supporting our theory.
The resulting difference map showed the absence of the pocket factor in both the 160s and 135s stages! Our theory was disproven; though we did find something intriguing from the difference map. There was evidence of a structural change in the area where the pocket factor had been previous to the capsid conformation in the 160s to 135s transition. We still think that the pocket factor, or at least the area surrounding it, is involved somehow in the conformational change that takes place.
This information has been shared with the virology community and has been met with some significant interest. We are hopeful that we remain close to discovering the truth concerning the role of the pocket factor, and the other factors involved in the viral genome delivery to host cells.
This project was an incredible learning experience for me. I dedicated roughly 10 hours a week for 10.5 months to this project. The techniques, principles, and methods involved in the project were all new and fascinating to me. Along with the techniques I also experienced much of the emotions involved in scientific research. There are times of tremendous excitement as well as those of defeat. Learning by research is a process of trial and error and requires patience. This is why Dr. David Belnap was an ideal mentor. He was patient and supportive to me as I learned.
I am thankful to Brigham Young University’s dedication to undergraduate education. I am thankful for the opportunity to learn in such an engaging manner. I am thankful for these opportunities that the ORCA grant provided. This project and the associations with faculty and peers it has created, has been the highlight of my undergraduate education.
