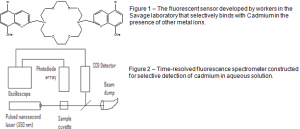
David Michaelis and Dr. Paul B. Savage, Department of Chemistry and Biochemistry
As society today continues to evolve technologically at a rapid rate, problems dealing with contamination of our natural resources become increasingly complex. Not only are new toxins introduced into the environment, but selective detection and removal of these contaminants proves ever more difficult. Heavy metals such as cadmium, lead, mercury, and radioactive nucleotides pose special problems to human health because of their tendency to accumulate in the body. Metal ions are specifically difficult to detect because of the large amount of benign ions (calcium, zinc, copper, sodium, etc.) that are naturally present in any water source. Fluorescence spectroscopy has made selective detection of metal ions a reality because of the potential of constructing organic sensors that selectively bind with metal ions of certain size. The Savage laboratory has developed an organic sensor with high selectivity for cadmium over other common metal ions (Figure 1). In preliminary studies, the surface sensor gave large fluorescent responses to cadmium at micro molar concentrations. However, the detection of cadmium in the presence of similar metal ions, such as copper and zinc, proved difficult. While these metals produce a significantly smaller response, the competitive effects make it difficult to measure cadmium concentrations with high accuracy.
The purpose of my project is to construct a time based fluorescent spectrometer that will enable selective detection of cadmium, dependant on the binding constants of the sensor for each metal. Because the binding constant of our sensor is greater for cadmium than any other metal, the fluorescent response due to binding with cadmium also lasts the longest. In time-based fluorescence spectroscopy, the sensor is excited with a short pulse of laser light, a short period (often several nanoseconds) lapses so that all other metal ion complexes and the uncomplexed sensor stop fluorescing, and the fluorescence of cadmium alone is measured. Comparison of the obtained response to a standard curve of response to concentration will allow us to determine the concentration of cadmium in solutions of various metal ions.
Our first goal was to construct a time-based fluorescence spectrometer capable of measuring time increments on the nanosecond scale. While commercial instruments are available, the cost of such instruments often outweighs their usefulness. We set out to show that a similar device could be constructed with simple commercially available instruments at a very small fraction of the price. As seen in Figure 2, a laser capable of creating nanosecond pulses is used to excite the sample containing the sensor. A small portion of the laser light is also reflected off a glass slide and collected by a CCD detector. The CCD detector acts as a trigger for the oscilloscope, telling it when to start and stop collecting data. The oscilloscope processes the data and displays it on the nanosecond scale. The photodiode array is the instrument that collects the signal from the sensor. Because the laser works on the nanosecond scale and the detector can process data in an equivalent fashion, this instrument makes it possible to detect even small changes in the sensor’s intensity by rapidly averaging a large numbers of scans.
In order to obtain accurate measurements from our time resolved spectrometer, the response time of each instrument has to be measured. Of particular importance is the photodiode array because it directly measures the sensor’s response to the metal ions and can easily give misleading information on the time scale we are working on. Through a series of reflection experiments using the laser light, we were able to determine the fall time of the photodiode to be well below the fluorescence lifetime of the sensor in the presence of cadmium (1 ns as compared to 30 ns). This meant that the measurements we obtained could be trusted for their accuracy.
Once the instrument was tested for its accuracy on the nanosecond time scale, we could begin to explore the time response of the cadmium sensor. I began by re-synthesizing the metal sensor, similar to the sensor in Figure 1, to be used in solution phase experiments. We then obtained a calibration curve that determined the response of the sensor at different concentrations of cadmium. To our excitement, we were able to measure the characteristic increase in the fluorescence lifetime of the sensor when complexed with cadmium. This data will allow us to compare the fluorescent response of the sensor to other similar metal ions. Selective detection of cadmium concentrations depends both on eliminating the background fluorescence of the unbound sensor and eliminating fluorescence of the sensor bound to other similar metals. These two factors are both addressed by the time-resolved fluorescence spectrometer we have constructed. We hope that the difference in binding constants for the similar metal ions and the elimination of background fluorescence will allow us to get selective detection of cadmium concentrations in any water source.
Testing will continue with our newly constructed time-resolved fluorescence spectrometer to determine how selectively cadmium can be detected in the presence of metal ions with similar properties and size. These studies will include competitive studies with the sensor to determine selectivity of binding and fluorescent response of the sensor in the presence of multiple ions. Our findings will then be published in a scientific journal in the field of inorganic chemistry.
One on the most valuable results of this project was the interdivisional cooperation in the chemistry department that was necessary for the project to succeed. I was able to work in conjunction with another research group in the analytical chemistry division (the group of Paul B. Farnsworth) both for instrumentation support and experimental design. This project helped me broaden my understanding of another area of chemistry and better appreciate how collaboration between the different areas of chemistry is not only beneficial but in many cases necessary if valuable research is to be conducted.