Amy Baker and Dr. Steve Turley
Abstract
The extreme ultraviolet (EUV) spectrum is becoming increasingly important. Its most promising applications include lithography for integrated circuits, space-based astronomy, and medical microscopes. Unfortunately, the optical constants of materials, particularly heavy metals, in this range are not well known. This work examines the molecular composition and oxidation rate and depth of thorium. Most of our data is collected through the use of X-ray Photoelectron Spectroscopy (XPS). XPS utilizes the photoelectric effect to obtain data about the exact composition of our material. X-rays are directed at the surface in question, colliding with and dispelling electrons from different energy levels of atoms. By measuring the number of electrons dispelled and their energies, the presence and quantities of elements can be determined. Depth profiling is done to examine the deeper layers of the sample, in which layers are etched off and new data is obtained. The results are then compared with existing literature. These methods are used to determine what chemical bonding occurs on the surface, whether or not it is diffused into lower layers, over what amount of time, and how the chemical composition varies with depth.
Introduction
Extreme ultraviolet, or EUV, technological applications include lithography, medical microscopes and space-based astronomy. The technological world relies on the use of lithography, where a silicon wafer is coated with a photosensitive material. When exposed to light, a pattern is created, similar to the way a photograph is taken. This method is used in integrated circuits, particularly computer chips. The technological world, however, in the attempt to increase computer speed and decrease computer size, is gradually switching to the use of extreme ultraviolet lithography. EUV light has high-energy and short wavelengths. This short wavelength allows the creation of smaller images. Hence, the computer chips become smaller and faster. With current lithography, we achieve a computer speed of 3-4 GHz. Through the use of EUV lithography, speeds of up to 25-30 GHz may be achieved.
EUV technology also has applications in the medical world. Current scanning electron microscopes are effective, but require extensive preparation. The microscope slides have to be coated with metals that significantly damage the sample. EUV microscopes require less preparation, thereby causing less destruction and allowing scientists to view the cells closer to their natural environment.
The principle application for EUV technology, particularly thorium, is space-based astronomy. Thorium shows promise as a durable, effective reflector in the EUV. If, upon further examination, thorium holds up as a valuable reflector, thorium could have significant applications in astronomy. With current technology, obtaining deep space images requires mirrors with very large radii. Through the use of satellites, several smaller mirrors, possibly constructed with thorium, can be positioned around the earth. By coupling the images from these smaller mirrors, a single image can be obtained. This yields the same result as a mirror with a radius the size of the earth’s would, allowing us to image deep space. The ultimate goal would be to examine deep space and observe rare astronomical phenomena, perhaps even black holes.
Theory
EUV has many applications in modern day technology. Unfortunately, the optical constants of materials in the extreme ultraviolet range are not well known. The term “optical constants” refers to the complex index of refraction, N = n + i k (1). Oxidation, which is negligible in the visual spectrum, has a significant effect on reflectance in the EUV range. The utilization of EUV technology requires a more detailed understanding of molecular composition and how heavy metals interact with light in the EUV range.
Thorium is an element of interest for many reasons. It only has one oxidation state, where it forms ThO2. This is convenient because it allows for more control over the molecular state of the sample. Oxidation cannot be prevented, but a better understanding of the molecular state allows for more accurate calculations of optical constants. Also, thorium is remarkably stable. It has the highest melting point of any known oxide at 2200 K, making it highly versatile. Calculations show that thorium should make a good reflector of EUV light (see Figure 1).
X-ray Photoelectron Spectroscopy (XPS) is a means to examine molecular composition. The main purposes for utilizing XPS are to determine the depth of the thorium oxide layer, to understand how the composition changes with depth, and to obtain an expression for oxidation as a function of depth. XPS utilizes Einstein’s photoelectric effect. When light shines on a metallic surface, atoms in the metal absorb quantized packets of light called photons and ejects electrons. XPS shines high energy EUV light on the surface in question, causing electrons to be emitted from the surface. The electrons are then sent into the analyzer, a magnetized chamber that detects the electrons’ final positions (see Figures 2 & 3).
Calculated Reflectance vs. energy (eV) at 5 deg
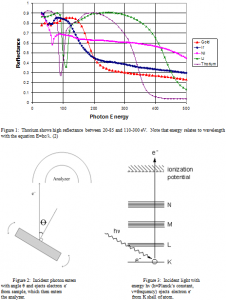
Based on the final positions of the ejected electrons, kinetic energy is calculated. The electron binding energy is then calculated using the equation Φ=hυ-Kmax (2), where Φ is the binding energy, or work function, hυ is the energy of the incident light, and Kmax is the kinetic energy of the ejected electrons.
The number of electrons hitting the detector (N) is proportional to the amount of element in the sample (n). The equation
![]()
is used, where ƒ is the x-ray flux in photons per cm2, σ is the photoelectric cross section, ө is the angular efficiency factor, ү is the efficiency in the formation of photoelectrons, λ is the mean free path of the photoelectrons, A is the area hit by the x-ray beam, and T is the detector efficiency. All of these components, excluding N and n, make up the relative atomic factor (S), making the equation.
Using the Equation4
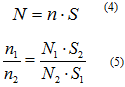
5composition can be determined.
Depth profiling is the method used to examine the lower levels of the sample. A literal hole is made in the sample by emitting, or rastering, a stream of argon ions across the sample under high vacuum, a process known as sputtering. The massive argon ions, having a large kinetic energy, effectively remove molecules from the sample. The depth at which it is scanned, or at which electrons and ejected and analyzed, is proportional to the density of the sample and the time spent sputtering.
Analysis
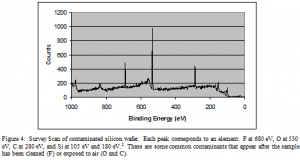
A graph, called a survey scan, is obtained of the number of electrons detected versus the binding energy in electron volts (eV). Some electrons collide or scatter on the way to the detector and consequently lose energy. These electrons form the background visible in Figure 4.
Results
Figure’s 10 and 11 are survey scans that have been amplified to view the thorium peaks better. The x-axis is energy in eV. The y-axis is the literal number of electrons detected.
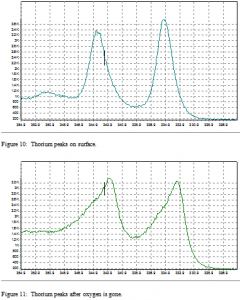
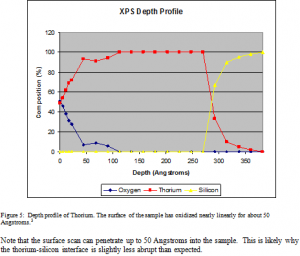
The change in peak shape is due to oxygen bonding on the surface. The peaks are thinner in the presence of oxygen. There is also a slight shift in energy level present. This shift is normally between 4 and 7 eV. In this case, the thorium peaks shift 2 eV higher in the presence of oxygen. This shows that the oxygen is bound to the thorium on the surface, and not simply resting on the surface due to contamination.
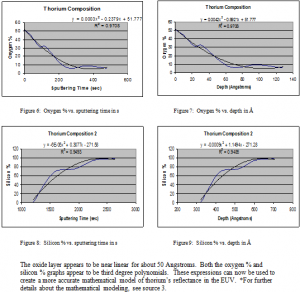
Although thorium is not generally considered to oxidize, the results show that thorium thin films do oxidize. Fortunately, the oxidation is found to be minimal. The thorium oxide layer is shown to extend about 80 Å down into the sample. About 10% of the sample is oxidized. These expressions for oxidation can now be used to more effectively mathematically model and study the reflectance of thorium. Thorium shows definite promise as an effective, durable reflector in the EUV.
Future Research
Future research will involve improving XPS technique. This will include examining specific sputter rates. The depth sputtered varies with the time spent sputtering, the mass and the density of the sample. However, there are other factors affecting the sputter rate. The shape that is sputtered is also significant. If the argon gun were to create a vertical hole, the sputter rate would remain constant as it sputters deeper into the sample. It is more likely, however, that a bowl shape is created. In which case, sputtering at the surface of the sample would be faster than sputtering deeper into the sample. The sample will need to be examined using Atomic Force Microscopy to obtain a model of what the sputtered area looks like. Future research will also involve examining the oxidation of thorium as a function of time to aid in understanding of storage and treatment procedures. This would simply require examining a sample at different ages. The oxidation of other potential EUV reflectors will also be examined.
