David Feller and Dr. Steven A. Fleming, department of Chemistry and Biochemistry
Cyclobutane rings occur in many natural products that show important medicinal properties (including sedatives, antiviral, and anti-tumor agents). The intrinsic physiological activities that cyclobutane rings display, draw the attention of synthetic chemists to elucidate total syntheses.1 Many natural products that incorporate chiral cyclobutane systems fascinate synthetic chemists as target molecules.
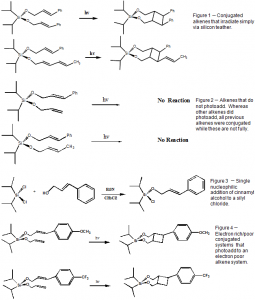
Previous research in our lab has shown that the silyl tethered alkenes in figure 1 react after irradiation, forming cyclobutane rings in a characteristic [2+2] fashion. The silyl-tethered alkenes in figure 2, however, did not photoadd. This indicates that where one photoaddition was favored, this one was not. The presence of an activated π system for one substituent could make the later photoaddition possible. Possible ways to activate a π system is to add electron withdrawing or donating groups to aromatic rings. Figure 3 shows p-trifluoromethylcinnamyl alcohol as an electron withdrawing system and p-methoxycinnamyl alcohol as an electron donating system.
The purpose of this project is to elucidate the mechanism of a [2+2] photocycloaddition of alkenes via a silyl-tethered molecule (a mixed dialkenoxysilane) and to understand the promoting effects of π -stacking or other π conjugated systems for [2+2] photocycloaddition. An increase in the utility of [2+2] photocycloaddition for synthesis will be made possible if the conditions are found to create cyclobutane rings from one aromatic (or π system) and a non-conjugated system.
The desired material to synthesize is a dialkenoxysilane, a silicon centered molecule. Silicon is tetravalent, and the synthesis of the dialkenoxysilane involves two alkenoxy groups and two alkyl groups. The alkyl groups are bulky to enhance alkenoxy interaction (Thorpe Ingold Effect). First, preparation of the alkenoxy substituents is necessary to react with diisopropyldichlorosilane. The targeted alcohols for synthesis include p-trifluoromethylcinnamyl alcohol and p-methoxycinnamyl alcohol. The carboxylic acids of these alcohols are commercially available, although expensive. Previous research found the reduction from the acid to the alcohol was difficult if not impossible. These acids were first esterified with ethanol at reflux temperatures for 10 hours. They were purified by chromatography and a yield of 85 percent was observed.
The esters were then reduced by two equivalents of DIBAL (diisobutylaluminumhydride) at 0° C. The reaction vessel was then let to warm to room temperature and reacted for 2.5 hours. The products were also purified by chromatography and characterized by NMR analysis. The reaction of the trifluoro involved a very colorful (yellow) intermediate until completion, which resulted then for both alcohols to be a fine white powder.
The alcohols were added to the diisopropyldichlorosilane under nitrogen gas (to prevent hydrolysis by water) as shown in figure 3. The reaction was run in methylene chloride with base (triethylamine) at reflux. Synthesis of mixed dialkenoxysilanes was also made. Instead of two equivalents of each alcohol, the same procedure was repeated for two separate alcohols, by addition of one equivalent of each alcohol. Synthesis of crotyl alcohol was also done, following the same conditions for previous alcohols.
Once a dialkenoxysilane was prepared, photo-irradiation was done by a 400 watt mercury lamp. The solution was approximately 0.5 mM in acetonitrile. The dialkenoxy first irradiated was the p-trifluorocinnamyl-crotyl-diisopropylsilane in figure 4. The reagent was irradiated for 2 hours.
The over-all synthesis of the reagent p-trifluorocinnamyl-crotyl-diisopropylsilane in figure 4 produced a low yield, approximately 15 percent. Synthesis of the ester was quite favorable, having a 95 percent yield. The reduction of the ester was overwhelmingly favorable, near 99 percent total yield. The step of the lowest yield was in figure 3. Synthesis of the alcohols to the dichlorosilane, for p-trifluorocinnamyl-crotyl-diisopropylsilane, was about 20 percent. NMR analysis of the crude product after roto-evaporation, showed no substantial product. No aromatic rings in the crude product were found and no further analysis was made.
It is believed that water was allowed into the system to hydrolyze the silyl chloride thus deactivating that silane for further use in preparation for a dialkenoxysilane. If one alcohol was allowed to add but water hydrolyzed the remaining chloride, then the whole alkenoxy and silane were made useless. Thus the yield for figure 3 was low.
Unfortunately, it was not possible to verify if the reaction in figure 4 took place. No starting material was able to be recovered. It is believed that the starting material decomposed on the glassware and was not even added to the photo-reaction vessel. The object of this reaction was to elucidate the effects of an activated electro-aromatic ring with an alkene for photoaddition (especially with an electron withdrawing group to induct an electron current flow). Previous aromatic rings with alkenes have not been seen to photoadd, see Figure 2. It had been shown earlier in our group that π stacking plays little effect in the ground state. Data obtained from an absorption spectra of the alkene monomers of p-trifluoromethylcinnamyl alcohol and p-methoxycinnamyl alcohol were taken and found in figure 7. The blue and red lines show the absorption of each alkene singly. The black line shows a calculated addition of the two lines and the green line shows the measured value of p-trifluorocinnamyl-crotyl-diisopropylsilane. The observation shows that no interaction is had between the alcohols and that π stacking does not exist for these two. Yet it was proposed if under irradiation and excitation, that an exciplex exists and that it would be sufficient for the reaction to in Figure 4 to occur.
It has been shown that two aromatic or conjugated π systems will photoadd while a conjugated system and an alkene will not. Data from absorption spectra for of p-trifluorocinnamyl-crotyl-diisopropylsilane show no sign of stacking and intersystem “talking.” Although it was hoped to elucidate an exciplex mechanism for this [2+2] photoirradiation, the results did not make it possible to ratify any hypothesis. Research continues in the production of p-trifluorocinnamyl-crotyl-diisopropylsilane and other dialkenoxy silanes to understand the mechanism of the cyclobutane addition and the effects of stacking and other factors.