Jennifer Mabey and Dr. Scott Steffensen, Department of Psychology
The aim of my ORCA grant was to better under the addictive pathway of ethanol (EtOH) in the ventral tegmental area (VTA) of the brain. The VTA contains several neuron types that release different neurotransmitters, but the type I experimented with was γ-Aminobutyric acid (GABA), which is the primary inhibitory neurotransmitter in the brain. GABA neurons regulate dopamine (DA) neurons in the VTA by inhibiting DA release but when the GABA neurons are inhibited, DA neurons are hyperexcitable and therefore have an increased DA release in the VTA. I originally sought out to see if nicotinic acetylcholine receptors (nAChRs) present on GABA neurons in the VTA is where EtOH is known to act (Davis and de Fiebre). Specifically I suspected that the nAChR subunit α-7, may be the leading mechanism of action of EtOH in the VTA. From previous experimentation in Dr. Steffensen’s lab, it was found that GABA neurons in the VTA are experiencing plasticity – changes in neuronal behavior – when exposed to only one injection of EtOH. I hypothesized that plasticity occurring in VTA GABA neurons could be blocked by the α-7 nicotinic antagonist methyllycaconitine (MLA).
Soon after I began to collect data, my mentor received an offer to collaborate with researchers from the University of Toronto on an addiction project regarding the VTA GABA neurons’ effect on opiate motivation. Because I had to yet to gain significant data that could have supported my hypothesis, my project was put on hold. Instead, I will report the results of the new project I worked on.
The dogma in the opiate addiction field is that mu-opioid receptors (MORs) located on VTA GABA neurons are activated by opioids leading to disinhibition of DA neural activity and release in the NAcc. A corresponding switch is observed in VTA GABA receptor subunit A (GABAAR) on VTA GABA neurons, which change from mediating hyperpolarization to mediating depolarization.
Chronic exposure to opiates increases BDNF levels in the VTA. The researchers at the University of Toronto found that brain-derived neurotrophic factor (BDNF) produces an opiate- dependent state and mediates the switch in GABAAR function in VTA GABA neurons. For example, the typical inhibition of VTA GABA neuron firing by the GABAA agonist muscimol is reversed by prior treatment with BDNF.
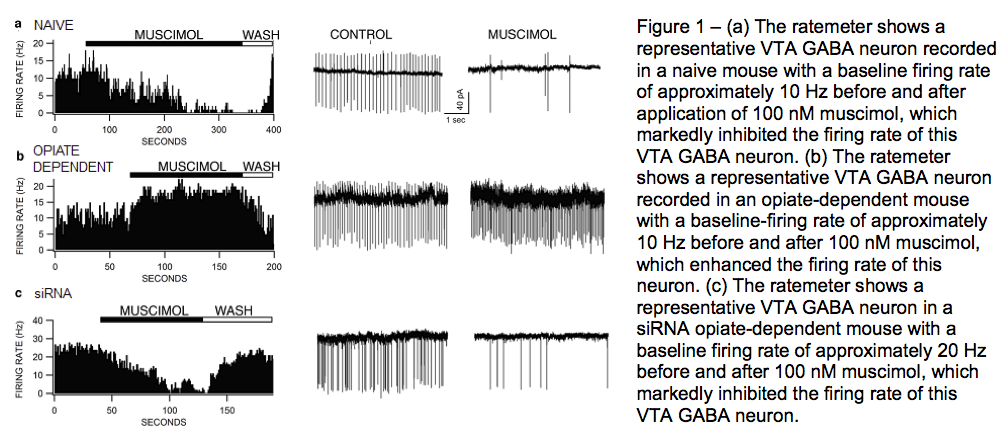
Using this knowledge from previous experiments, we hypothesized that knocking out BDNF tyrosine receptor kinase B (TrkB) receptors with TrkB small interfering RNA (siRNA) in the VTA will block opiate dependence and restore the inhibitory effects of the GABAA R agonist muscimol on VTA GABA neuron firing rate. siRNA acts by interfering the expression of specific genes, in this case the BDNF TrkB receptors (Vargas-Perez H et al.).
To test this, I performed experiments using cell-attached patch clamp electrophysiology, where I recorded spontaneous GABA firing activity in drug free control mice (naïve), opiate dependent mice, and mice injected with siRNA that knocked out BDNF TrkB receptors. Neurons were voltage-clamped at 0 mV throughout the experiment. First, a stable baseline recording of firing activity was obtained for 5 min. Then 10nM muscimol was applied to the bath for 5 min. The muscimol concentration in the bath was increased to 100nM for another 5 min, followed by a final increase to 1μM muscimol. This was done to find the dosage that muscimol had the greatest effect on neuronal firing rate. 100nM muscimol was found to be the best. The results for GABA firing rate for naïve, opiate dependent, and siRNA injected mice with 100nM muscimol are shown in Figure 1.
These results show that muscimol significantly inhibited firing rates in naive and siRNA mice, but not in opiate dependent mice. Depletion of TrkB receptors in the VTA prevents the switch in GABAA R function in VTA GABA neurons by chronic opiate treatment, suggesting that these neurons, which express MORs, are critical adaptive neuronal substrates underlying opiate dependence.
The poster for this ORCA projected was presented at the University of Utah’s Annual Snowbird Symposium in fall of 2012 (Mabey et al.).
References
- Davis TJ, & de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006; 29(3):179-85.
- Vargas-Perez H, Ting-A-Kee R, Walton CH, Hansen DM, Razavi R, Clarke L,
- Bufalino M, Allison DW, Steffensen SC, van der Kooy D. (2009). Ventral tegmental area BDNF induces an opiate-dependent–like reward state in naïve rats. Science. 324(5935):1732-4.
- Mabey JK, Shin SI, White D, Nielson C, Vargas-Perez H, Ting-A-Kee R, Bahi A, van der Kooy D, Steffensen SC. Ventral tegmental area GABAergic activity underlies opiate motivation. Poster presented at University of Utah Snowbird Symposium Fall 2012. 2012, Nov 2-3; Snowbird, Utah.