Cheryll Garn
Abstract
This study investigates whether males and females use distinct brain systems while performing a picture naming task. Previous neuroimaging studies have shown that in some cognitive processing domains, such as spoken language comprehension, males and females differ in terms of brain hemisphere activation, with a tendency for stronger left hemisphere lateralization in males. The purpose of this study is to test whether an analogous hemispheric difference (or other systematic difference) might be found between males and females in a picture naming task, which includes visual and verbal processing components. Brain activation differences were assessed using functional magnetic resonance imaging (fMRI) data from fourteen participants (seven males and seven females) between the ages of 18 and 30. Results did not confirm the expected hemispheric asymmetry. However, females showed increased activation in left thalamus, bilateral anterior insula/frontal opercula, left pre-motor areas, and medial prefrontal cortex including the cingulate gyrus compared to males. Though unanticipated, these observed differences provide support for a more explicit theory of sex-differences within a multi-staged model of object recognition and naming that parsimoniously tie together findings of previous reaction time studies that examined sex-differences in picture naming.
Background and Introduction
With the recent advances in imaging technology during the last several decades, researchers have been able to study how the brain works when it is engaged in perceptual and cognitive processing. Using functional imaging techniques, researchers are able to obtain continuous samples of brain activity as often as every two seconds while participants are engaged in a cognitive task, such as viewing pictures or listening to a recording. Functional Magnetic Resonance Imaging (fMRI) is a recent imaging technique that allows experimenters to assess brain function by measuring changes in blood and oxygen levels in real time as the subject performs a given task. By observing these changes, inferences can be made regarding the neural areas activated while participants perform a cognitive task. While recent studies have used functional imaging to assess how the brain processes perceptual stimuli and performs a variety of cognitive tasks, only a few studies have looked specifically at sex differences.
Sex differences
Sex differences have been found in language comprehension tasks with fMRI. Phillips et al. (2001) found activation in females in the right and left temporal lobes while participants listened to an auditory stimulus (a recording of someone reading), whereas neural activation in males was restricted to the left temporal lobe. The extent of activation across the left temporal lobe was significantly greater for males than that observed in females for either hemisphere. Although research such as this has shown measurable sex differences, it is unclear if these or similar differences can be seen for other cognitive processes.
With the use of electroencephalograph (EEG), research has assessed sex differences in neurocognitive functioning. The EEG data can determine comprehension speed, and more recently, can determine which parts of the brain react to a stimulus first. An EEG study that focused on sex differences found two different patterns of brain waves in males and females as a function of age (La Marche et al., 1986). The older females produced a larger wave pattern than the males and younger females, showing increased activity after being presented with patterned and unpatterned flashes of light. These results suggest that males and females differ in sensitivity and in some neural activity to visual stimuli, especially with increased age.
Sabatinelli et al. (2004) had college-aged males and females view a variety of pictures that were created to evoke emotional and neutral responses. The males and females had similar activation when viewing familiar or neutral pictures, but males had a greater BOLD signal change when viewing erotica compared to females, and females had greater neural activity than males when viewing emotionally aversive pictures. Their research suggests that there are sex differences in neural activity when the emotional content of visual stimuli varies. However, it is not known whether males and females might differ in BOLD response for neutral pictures vs. a non-picture baseline.
A study by Laws (2004), that assessed sex differences in picture naming speed, had participants view pictures from four separate categories: animals (living), fruits (living), tools (nonliving) and vehicles (nonliving). He found that females identified living items more quickly than males and males named nonliving items more quickly than females. Three other experiments in this study showed similar results. Laws (2004) study demonstrates a difference between the recognition and naming of an object. The object of this study is to find out what the brain processes are that can account for the difference Laws found.
Visual processing and Picture naming
Visual Analysis. Picture naming includes three stages: visual analysis, object recognition, and name retrieval. In this paper I will refer to visual analysis as a ‘lower level’ process, and object recognition and name retrieval as ‘higher level’ processes.
Visual information is received through the eyes and transmitted to the back of the brain, or the occipital lobe. This visual input is first analyzed in the primary visual cortex, and then projects through two main pathways to the ‘dorsal’ and the ‘ventral’ visual systems (see figure 2). In the dorsal visual pathway information travels from the occipital lobe and the primary visual areas into the parietal lobe. In the parietal lobe sensory input from many neural regions comes together to determine the spatial location of a stimulus. For example, it is used to locate where a book is in space so, if needed, a person could easily grab it. Hence, the dorsal pathway is also referred to as the ‘where’ pathway since the brain determines where an object is in the visual field. (Haxby, et al., 1991). The other pathway, the ventral visual pathway, takes visual information from the occipital lobe into the temporal lobe. The picture stimulus is then compared to representations stored in memory until an object has been recognized. This pathway can also analyze spatial relations within objects in order to better understand each object. Because it is in the temporal lobe where we reach recognition, the ventral visual pathway is also known as the ‘what’ pathway (Haxby, et al. 1991).
Object Recognition.
Focusing on picture recognition and the two main pathways of visual processing, Kraut et al. (1997) performed an fMRI study in which subject viewed line drawings of real objects and non-real drawings. They found that activation for object shape recognition is isolated in the visual system in normal subjects and that activation was also seen in both the dorsal and ventral pathways during shape recognition. The authors suggest the activation in the left temporal region was related to semantic meaning of objects, which is a necessary component of picture naming.
Name Retrieval.
The final stage of picture naming is name retrieval. Areas that may be activated in association with the name retrieval stage include the left inferior frontal gyrus and the anterior cingulate gyrus. The left inferior frontal gyrus (LIFG) is located in the left frontal lobe, near Broca’s area. The LIFG is used when retrieving information from long-term memory, like picture names. The anterior cingulate gyrus is located in the medial (central) area of the brain, just above the corpus callosum (connector between the two halves of the brain). The cingulate gyrus receives input from different areas of the brain and can be activated with increasing demands of attentional processing related to task difficulty (Paus, Koski, Caramonos & Wetbury, 1998; Posner & Dehaene, 1994). Activation of the anterior cingulate gyrus likely will occur during picture naming, especially with increasing task difficulty. Finally, name retrieval should engage areas associated with speech articulation in the left motor and pre-motor cortex
The three hypothetical stages of object naming, visual analysis, object recognition and name retrieval, and the brain areas associated with each of these stages are summarized in figure 3.
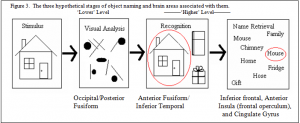
Areas of expected activation in picture naming tasks include occipital, fusiform, temporal and the speech motor areas. The speech motor area includes pre-motor and primary motor cortex in the left hemisphere which is located in the frontal lobe just in front of the central sulcus. Activation of the speech motor area is expected in picture naming tasks as this area of the brain tends to be activated when subjects covertly respond to a visual stimulus. The fusiform gyrus is an important structure in the ventral visual pathway and is located in the temporal lobe. It is used in color, face, name, and word recognition and is expected to be activated in this study.
fMRI studies of lower vs. higher level visual processing. Regarding lower-level visual processing, Cowan et al. (2000) used fMRI to investigate how males and females respond differently to varying intensities of red and blue light. They found that the BOLD signal change was similar in males and females to red light, but males had increased activation for blue light. In addition the slopes of stimulus-intensity vs. BOLD signal change curves were significantly lower in females for blue light stimulation. These results suggest sex differences in terms of light sensitivity.
Other neuroimaging studies have also focused on lower-level visual processing. One study looked at sex differences when the visual stimulation was a flashlight. Hedera et al. (1998) found that 35% of male participants had no detectible BOLD signal changes during the presentation of the visual stimulus (the flashlight), but 100% females showed strong MRI signal changes, indicating drastic sex differences. Another study using Positron Emitted Tomography (PET) and sustained checkerboard stimulation (in which a checkerboard pattern is continuously displayed as the stimulus) showed that “rCBF increase was significantly higher in females compared to males” (Kastrup et al., 1999, p. 1066) which also suggests sex differences in lower-level visual processing.
No fMRI study, that I am aware of, has focused on sex differences in higher-level visual object processing. One ‘influential’ study that has contrasted lower vs. higher processes, but which did not make a comparison between the sexes is Kanwisher et al. (1997). They looked at object recognition by comparing brain responses to familiar, pseudo and scrambled line drawings in an attempt to localize the lower vs. higher level component processes of object recognition. Because Kanwisher et al. (1997) were not interested in the name retrieval component of higher level processing, the pictures were displayed very quickly such that participants could recognize them, but not have time to produce their names. The real pictures were line drawings of common and easily recognizable manmade objects: for example, a simple drawing of a piano or wagon (see figure 5a.). The pseudo pictures (see figure 5b.) were designed to have many visual features of real objects, so that they would likely be treated as possible real objects at early processing stages, but fail to access any stored representations in memory at later stages of processing. The scrambled objects were created from familiar pictures that were taken apart and, in contrast to the other two variables, did not have any features that would make them recognizable as real objects (see figure 6c.). The authors did not look for any sex differences in fMRI activation between familiar and pseudo objects, they did find neural activation for both familiar and pseudo objects differed from that of scrambled objects. The familiar and pseudo pictures elicited significantly more neural activation than the scrambled pictures in fusiform and inferior posterior temporal cortex, especially in the right hemisphere.


These areas of the brain (the fusiform and inferior posterior temporal cortex) are thought to be involved in higher-level visual form processing. Kanwisher et al. (1997) theorized that in the familiar and pseudo conditions, the brain reacts to the visual stimuli and then tries to search for a match for the object within memory, whereas no similar search is carried out in response to the scrambled stimuli.
The present study was designed to extend the findings of Kanwisher et al (1997). Line drawings similar to the ones used by Kanwisher et al., with similar contrasting conditions (the familiar and scrambled conditions), were presented to subjects, but with an additional experimental comparison of sex. Given that right vs. left hemisphere sex differences have been found in auditory processing (and other sex differences have been found in lower level visual processing) it is possible that a hemispheric difference (or some other difference) might be found in the way males and females process higher level visual information, including object name retrieval. The purpose of this study was to test for sex difference in brain activation in a picture naming task where participants are allowed enough time to fully complete the naming process.
Methods
Participants.
Fourteen participants (7 female, 7 male) between 18-30 years old were recruited for the study. Participants were recruited via the social, academic, and family networks of the principle investigator. Since data collection and analysis are expensive and time consuming in fMRI research, a high level of participant comfort and compliance is required. In Dr. Allen’s (2004) experience, people who are already somewhat acquainted with the Functional Neuroimaging Lab and its members (e.g., fellow faculty, student research associates, family, and friends) have met this requirement the best and invitations to participate were made informally. Participants did not receive compensation.
General Procedure.
Each participant viewed a number of simple pictures while in the fMRI scanner. The visual stimuli were projected on a screen that was placed in front of the fMRI machine. The participants were able to view the stimuli with the assistance of two mirrors angled in a way that allowed them to see the screen. The visual stimuli included familiar and scrambled line drawings, modeled after those of Kanwisher et al.’s experiment (1997). Change Figure 5a. gives examples of the familiar pictures used, and figure 5c. gives examples of the scrambled pictures used. During the procedure, the participants were shown a total of 60 drawings. These included 32 familiar and 28 scrambled pictures. Unequal numbers of familiar and scrambled pictures were used because of common procedure for the number of study sessions. Each drawing stimuli was shown for one second. Due to the limitations on the computer program for fMRI scan acquisition, only four pictures were shown sequentially during each block (each block was 24 seconds long). Each block began with an eight second blank period before and a twelve second blank period after the presentation of the four pictures. Each participant viewed four familiar pictures in the first block, four scrambled pictures in the second block, four familiar pictures in the third block, and so on. Five sessions of three blocks were presented to each participant.
fMRI Methods
Anatomical Scanning. Before the actual fMRI experiment took place, a pre-scan of the participant’s brain was done. From the 16 sagittal slices acquired, the anterior (AC) and posterior comissures (PC) were identified in the midsagittal slice as landmarks for aligning the functional slices. High-resolution 3D structural brain images were acquired from each subject using a T1-weighted spoiled grass gradient recalled (SPGR) sequence at the end of a session with each participant.
Functional MR Scanning.
Twenty-four contiguous 4-mm slices of functional images (covering the whole brain) were acquired during each two second period from participants using gradient-echo echoplanar images (EPIs) sensitive to blood-oxygen level dependent (BOLD) contrast with a 2-second TR , 31-ms TE, one radio frequency excitation, a 90 flip-angle, a 24-cm FOV with a 642 image matrix resulting in a 3.75-mm2 in-plane voxel resolution.
Data Analysis
Data preprocessing and analysis was carried out with the SPM2 statistical parametric mapping software package (Wellcome Department of Cognitive Neurology, London, UK. http://www.fil.ion.ucl.ac.uk; Friston, Holmes, Worsley, Poline, Firth, & Frackowiak, 1995).
Image Preprocessing. Prior to statistical analysis, the first volume of each block was discarded due to T1 susceptibility while the remaining functional volumes from each participant were submitted to a slice acquisition-time adjustment procedure, using sinc interpolation, so each of the 24 slices in a volume (collected over 2 seconds) were re-set to the same acquisition time. To account for head movement during the experiment, functional volumes were realigned to the first volume of the sequence using a 6 parameter rigid-body affine transform (the 6 parameters are in 3 translation and 3 rotation directions) and corrected for EPI distortions. The volumes were then resliced with a 4th degree b-spline interpolation function. A mean volume image was also created using the time- and motion-corrected volumes. Then, the anatomical (structural; SPGR) for each participant was coregistered to his/her mean functional image.
Anatomical and mean functional images were then coregistered and spatially normalized (so that each volume would look similar) to a standard stereotactic space using the Montreal Neurological Institute (MNI) templates implemented in SPM2. The spatial normalization parameters applied to the mean functional images for each participant were then used to spatially normalize all of the realigned functional images for that participant. All functional volumes were resampled to a 3mm3 voxel size and spatially smoothed with an 8mm full width at half maximum (FWHM) isotropic Gaussian kernel . This was done to increase signal-to-noise ratio and to reduce the effects of moderate intersubject variability in brain anatomy.
Statistical Analysis.
A boxcar waveform convolved with a synthetic hemodynamic response function (HRF) with a 4 second lag-to-peak was used as a reference waveform for each of the two within group conditions (Real vs. Scrambled). The data were high-passed-filtered in time, using a set of discrete cosine basis functions with a cut-off period of 128 seconds. This was done to remove low frequency noise due to such things as breathing cycles and temperature changes within the scanner.
Within group (sex) condition effects were estimated for each voxel over time according to the general linear model (ANCOVA) with familiar and scrambled as condition variables and global activity (e.g., session effects, magnetic field drift) as a confounding covariate. BOLD signal changes for each voxel assessed relative to the average signal across the whole brain at each time sample. The resulting foci of activation were characterized in terms of peak height (the highest point of activation) and spatial extent (the activation with largest special extent). Regions of significant activation were then identified and coded in terms of the resulting t-statistic for each voxel that exceeded the significance threshold of p < 0.001 and that was part of a cluster that included at least 8 contiguous suprathreshold voxels.
Contrasts between the conditions familiar vs. scrambled were examined with scrambled as the control condition. Differences between these conditions were estimated first for both sex groups pooled together as well as within each sex group, by subtracting the activation of the control condition (scrambled) from the experimental condition (familiar). Each contrast was created using a statistical masking technique developed in SPM so that only positive activation of the familiar condition, rather than deactivation in the scrambled condition, was shown.
Between group contrasts were evaluated by overlaying activation of familiar vs. scrambled conditions in a single display. Inferences were only drawn from activations where a clear difference was seen (specifically, activation for one group did not overlap with the other), and not for areas of overlap. Between group (sex) contrasts statistics were not done since it would be impossible to distinguish areas of clear difference from those of partial overlap. This highly conservative approach was adopted because of the overall low number of participants and observations per condition (i.e., low power), which is a common limitation in fMRI research.
Results
Neuroactivation with Familiar minus Scrambled Picture Stimulus for all Participants
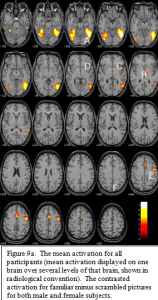
The contrast for familiar pictures minus scrambled pictures (which only isolate the mean activation for familiar pictures) across both sex groups is displayed in figure 9a. The peak activation occurred bilaterally in the fusiform gyri,A, extending into the lateral occipital gyri. In the left
hemisphere this posterior ventral activation source further extends dorsally into posterior temporal and lateral occipital areas, with a center of focus on the lateral occipital sulcus. Other areas of significant activation include bilateral thalamus,B, bilateral anterior insula/frontal opercula,C, left inferior frontal gyrus (Broca’s area),D, left and medial pre-motor cortex, E, and the left parietal lobe in the region of the interparietal sulcus.F,
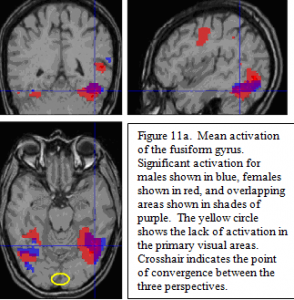
Neuroactivation for Familiar minus Scrambled Pictures Stimulus between Groups
Individual comparisons for real minus scrambled pictures revealed distinct patterns for sex groups. The areas of common activation are in the posterior regions of the ventral visual object processing pathway (fusiform gyri and left posterior inferior temporal/lateral occipital areas ; figure 11a). Note the lack of activation in primary visual areas (see circle in 11a), due to the fact that activation in the lower level visual areas common to both real and scrambled pictures was subtracted out in the statistical contrast.
The contrast between sexes revealed the activation for females included the ventral visual areas that were seen in the males, in addition to other language processing areas not seen in males. These additional areas include left thalamus, the anterior insula/frontal opercula, the left pre-motor areas, and medial prefrontal areas centered around the cingulate sulcus. Additionally, there was a highly significant activation for females in the left intraparietal sulcus, as well as a much smaller activation on the right.
Discussion
I set out to find if males and females differ in neural function when processing visual stimuli and naming pictures. Specifically, I set out to test the hypothesis that there would be hemispheric asymmetry differences between the sexes. Although for visual processing and picture naming no sex differences were found in hemispheric asymmetry, the results demonstrate that females had activation in four other brain areas where males do not.
The data showed that males and females both had activation occur in the visual and language areas. These results are different than that of Kanwisher et al. (1997), who found activation of the fusiform and inferior posterior temporal cortex, especially in the right hemisphere. The results of this study likely differ from those of Kanwisher et al. (1997) because of variation in methods. One possible explanation is that stimuli used in Kanwisher et al.’s (1997) study were presented too quickly for the participants to name, resulting in identification and not name retrieval.
My original hypothesis was tested and between sexes I found that in the early visual areas (e.g. fusiform and lateral occipital cortex) there were no significant sex differences. However, in later processing areas, in particular those that relate more to language production than visual recognition, activation was found only in the female participants. The results of both Kanwisher et al.’s (1997) study and this study highlight the interesting disassociation between the processes of object identification and verbal name retrieval, as well as an interaction in the degree of involvement of these two processes between the sexes.
There are two possible explanations for the sex differences in activation with picture naming. The first is that the activation seen in females and not males may support the supposition that females are more focused on linguistic aspects of the stimuli. However, this explanation appears somewhat paradoxical since in a variety of verbal tasks women show superior performance (Halpern, 2000). The second possibility for a sex difference is quite the opposite and, instead, it would suggest that it is due to females having more difficulty in naming the stimuli. Increased activation has often been found to correlate with greater difficulty in completing a task in fMRI studies (Kan and Thompson-Schill, 2004).
There is a way to reconcile these two seemingly opposite explanations, by looking at the picture stimuli used. Another fact to consider is that females have worse performance for naming objects in certain domains. For example, females have been found to differ from males in terms of naming objects that can be manipulated by the hands and Laws (2004) found that males name pictures of tools significantly faster than females. The examination of the stimuli revealed that the majority of my stimuli were either tools (e.g. hammer) or manipulable objects (e.g. piano, hat, shirt etc). Another possibility is that since females have a richer vocabulary, the problem is with name agreement and choice. Kan and Thompson-Schill (2004) have shown that there is greater fMRI activation in the cingulate gyrus when there are more names to choose from. This occurs “when low agreement items, such as blouse, are named, more semantic retrieval is expected, because semantic cohorts, such as shirt and top, are also retrieved” (p. 54). Another suggestion is that women show more activation in language production areas, because they draw on more neural areas to accomplish the task, when it is comprised of manipulable objects or objects with low name agreement.
A further underlying question is when during the processing of picture naming do women encounter more difficulty. The three-stage model of visual processing shows that there is a distinction between identifying or recognizing and retrieving the name of an object. Thus it seems that the female participants in the study seem to have greater difficulty in the last stage, or name retrieval.
Additional unpublished research by Allen and Lewis (2004) gives further information in reaction time data with a picture stimulus. Subjects were simply asked to distinguish real objects from nonsense objects (similar to the pseudo objects used in Kanwisher et al.’s (1997) study; see figure 5b.) and were required to press a button whenever they saw a familiar object. Subjects were only asked to find a positive match to the visual stimulus with those stored in memory (see figure 11). Allen and Lewis (2004) found no sex differences in this object recognition task, which suggests that the additional time for female participants in Law’s (2004) study, and the additional activation seen in the present fMRI study is used for name retrieval (see figure 12).
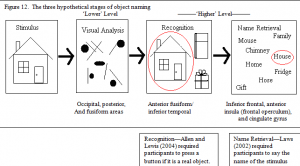
The fMRI results of this study further support the conjecture that females have greater difficulty in retrieving the names of the objects. The activation differences seen between the sexes are not in the ventral object recognition pathway, but are found in the areas associated with name production (frontal areas) and name selection (cingulate gyrus).

The main benefit of this study is that it assesses findings of previous studies for both neuroimaging and behavioral analysis of sex differences in picture naming by shedding light on specific brain functions and how these functions relate to the between-sex differences observed in prior object naming reaction time studies. The process of naming a picture, as presented in this thesis, is not a simple unitary procedure. Instead it requires a complex, multistage series of cognitive events. One limitation of reaction time studies is when between-condition differences (e.g. male vs. female) are found in a multistage cognitive process, it is difficult to determine which cognitive stage is responsible for the difference.
Incorporating neuroimaging data allows one to take advantage of a large body of knowledge regarding the association of brain areas with known cognitive functions. The advantage of doing a functional imaging study in conjunction with behavioral studies, allows correlations in areas of known cognitive roles with specific stages within the multistage process of object naming. In the case of this study, the distinct patterns of activation I found provided evidence needed to determine whether sex differences in reaction times were the result of visual analysis, object recognition or picture naming. The fMRI data demonstrate that the last stage, name retrieval, is the source of the observed sex difference.
A final question concerns the nature of the apparent disadvantage females have during this final stage. Is it because females have less efficient name retrieval mechanisms? The current fMRI data together with a meta-analysis of reaction time studies suggest that it is not due to inefficiency. Instead, the most likely hypothesis for increased activation in females is the tendency for females to have larger vocabularies, and therefore a larger variety of names to select from when confronted with a common visual object. That is, females become more susceptible than men to the effects of low name agreement (Kan & Thompson-Schill; 2004).
However, further studies are needed to strengthen this hypothesis. For example, it would be important to run a study that directly contrasts neuroactivation for natural and manmade objects within females, since it is assumed (based on Laws, 2004) that the increased activation for females during the name retrieval stage is specific to manmade objects. If this hypothesis is true, one would expect females to show greater activation in frontal areas, specifically in the anterior cingulate gyrus, when viewing manmade versus non-manmade objects.
A weakness of the current study is low statistical power. As mentioned in the analysis section, because of this relatively low power, we adopted a very conservative statistical approach. This approach precluded more fine-grained statistical comparisons in areas of partial overlap between males and females. There are two reasons for the low power: first, due to some current software limitations, we were not able to collect as many trials from each subject as would have been optimal. Second, because of the expense of running many subjects, we were only able to test 7 subjects in each condition. Although this low number of subjects is common in many fMRI experiments, larger sample sizes are needed for comparisons in areas of partial overlap without at least 20 subjects in each condition with improved fMRI software.
Conclusion
Although the results of this study do not support the original hypothesis of sex differences in hemispheric asymmetry, other significant differences between males and females were found. Specifically, females had increased activation in areas associated with name retrieval (including left thalamus, anterior insula/frontal opercula, left pre-motor areas, cingulate sulcus and left intraparietal sulcus). Furthermore, behavioral studies have shown that while males and females do not differ in terms of object recognition, they do differ in terms of object naming. Specifically, females take longer to retrieve object names for manmade items.
References
- Allen, M.D. (2004). Personal Interview.
- Allen, M. D. & Lewis, C. (2004). Unpublished Study.
- Cowan, R.L., Fredrick, B.de.B., Rainey, M., Levin, J.M., Maas, L.C., Bang, J., Hennen, J., Lukas, S.E., & Renshaw, P.F. (2000). Sex differences in response to red and blue light in human primary visual cortex: a bold fMRI study. Psychiatry Research: Neuroimaging Section, 100, 129-138.
- Friston, K., Holmes, K., Worsley, J., Poline, C., Frith, C., & Frackowiak, R. (1995). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2, 189-210.
- Halpern, D. (2000). Sex differences in cognitive abilities. Lawrence Erlbaum, Mahwah, NJ.
- Haxby, J., Grady, C., Horwitz, B., Ungerleider, L., Mishkin, M., Carson, R., Herscovitch, P., Schapiro, M., & Rapoport, S. (1991). Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Science, 88, 1621-1625.
- Hedera, P., Wu, D., Collins, S., Lewin, J. S., Miller, D., Lerner, A. J., Klein, S., & Friedland, R. P. (1998). Sex and electroencephalographic synchronization after photic stimulation predict signal changes in the visual cortex on functional MR images. American Journal of Neuroradiology, 19, 853-857.
- Kan, I. & Thompson-Schill, S. (2004). Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive, Affective, & Behavioral Neuroscience, 4(1), 43-57.
- Kanwisher, N., Woods, R.P., Iacoboni, M., & Mazziotta, J.C. (1997). A locus in human extrastriate cortex for visual shape analysis. Journal of Cognitive Neuroscience, 9(1), 133-142.
- Kastrup, A., Li, T. Q., Glover, G. H., Krüger, G., & Moseley, M. E. (1999). Sex differences in cerebral blood flow and oxygenation response during focal physiologic neural activity. Journal of Cerebral Blood Flow and Metabolism, 19, 1066-1071.
- Kraut, M., Hart, J., Soher, B.J., & Gordon, B. (1997). Object shape processing in the visual system evaluated using functional MRI. Neurology, 48(5), 1416-1420.
- La Marche, J.A., Dobson, W.R., Cohn, N.B., & Dustman, R.E. (1986). Amplitudes of visually evoked potentials to patterned stimuli: age and sex comparisons. Electroencephalography and clinical Neurophysiology, 65, 81-85.
- Laws, K. R. (2004). Sex differences in lexical size across semantic categories. Personality and Individual Differences, 36, 23-32.
- Paus, T., Koski, L., Caramanos, Z., & Westbury, C. (1998). Regional differences in the effects of task difficulty and morot output on blood flow response in the human anterior cingulated cortex: a review of 107 PET activation studies. Neuroreport, 9(9), 37-47.
- Phillips, M., Lowe, M., Lurito, J., Dzemidzic, M. & Mathews, V. (2001). Temporal lobe activation demonstrates sex-based differences during passive listening. Radiology, 220, 202-207.
- Posner, MI., & Dehaene, S. (1994). Attentional networks. Trends in Neurosciences, 17(2), 75-59.
- Sabatinelli, D., Flaisch, T., Bradley, M. M., Fitzsimmons, J. R., & Lang, P. J. (2004). Affective picture perception: sex differences in visual cortex? NeuroReport, 15(7), 1109-1112.

