Megan M. Woodhouse and Dr. Thomas Fletcher, Chemical Engineering
Empirical models have been developed to predict the spread of forest fire models from data about the burning plant species, terrain, and wind conditions. Over the past three years, a research group at Brigham Young University has collected data for ignition temperatures and time to ignition for eight leaf species involved in wildland fires. Four of these species are found in the Southern California chaparral, while the four other species are found in Utah. To further improve the fire models it is desirable to know the heats of combustion for each of the different leaf species. Heats of combustion can possibly be used to explain the ignition and burning characteristics of different leaf species.
Before the heat of combustion for a sample can be measured, the leaf samples must first be collected, dried, finely ground, and pressed into pellets weighing approximately 0.2 grams each. The Utah leaf samples were collected from Rock Canyon, Provo, UT during the summer of 2005. The species of interest are canyon maple, gambel oak, sagebrush, and juniper. Samples were dried using a CompuTrac moisture analyzer which heats samples so all moisture is removed. Then several leaves of each species were ground using a mortar and pestel and an electric coffee grinder. Pellets were made from the powdered leaves using a hydraulic press. Pellets of compressed leaf material were then used for the actual heat of combustion measurements.
Heats of combustion were measured for the four Utah species using an oxygen bomb calorimeter . Arrangements were made to use the calorimeter in Dr. Lee Hansen’s lab in the Chemistry Department. Four to five measurements were taken for each species. Measurements consisted of a series of temperatures recorded over a period of 1000 seconds while combustion occurred. The measurements were imported into Microsoft Excel to be calculated into a heat of combustion. I wrote a macro, or program, to automatically perform the heat of combustion calculation within the template given to me by Dr. Hansen’s graduate student. This program decreased the computation time by approximately 73%.
For each species an average heat of combustion was obtained from the four to five samples burned. Statistical analysis was performed for each species. The results are summarized in Table 1 below.
Original gambel oak measurements were the least reliable of the four species. This was due to using two different “bombs” for the measurements. The experiment was repeated with the equipment used in all other reported experiments. The results from the repeated experiments are shown below.
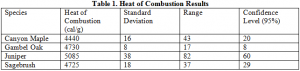
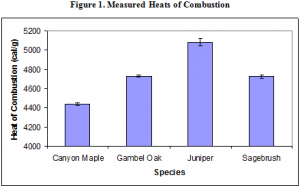
The other results appear to be quite precise. The heats of combustion determined for Juniper and Sagebrush are fairly consistent with measurements taken by White and coworkers using a cone calorimeter. The values Weise obtained for juniper and gambel oak were 4469 cal/g and 3394 cal/g respectively. These results are lower than the results presented in this paper, but that is expected given that a cone calorimeter does not burn all of the combustible material available, whereas a bomb calorimeter does. The results are further summarized in the following chart. Juniper has the highest heat of combustion, while Canyon Maple has the lowest.
The heat of combustion data that has been compiled can be used to predict flame temperatures, flame heights, and the rate of spread for wildland fires. Further research is being conducted to measure the heats of combustion for four plant species from California (scrub oak, manzanita, chamise, and ceanothus). These species were shipped to BYU during the summer of 2005, at which time they were dried and made into powder. It is expected that complete data will be available for the heat of combustion of these species by April 2006. Future research may include how the heat of combustion varies with moisture content or season.
