Taylor Turner and Dr. William Pitt Chemical Engineering
Introduction
As part of a constant pursuit of better health researchers continuously investigate the role of bacteria in disease and infection. Much research today investigates methods for effectively killing unwanted bacteria in the body. Modern antibiotic medications are the most effective method of accomplishing this, but they have their limits. One particular limit of today’s antibiotics is the ability to eliminate biofilms. Biofilms consist of many layers of bacterial cells growing on top of each other, typically attached to a solid surface. Antibiotics cannot penetrate the layers of biofilms, leaving the lower bacteria untouched after treatment. This problem led us to investigate the possibility of electricity as an effective antibiotic that could function in the place of, or alongside, traditional antibiotics. Our preliminary results indicated that it was possible to kill bacteria with low doses of electricity, but only in specific conditions. Here I report further investigation of those conditions and the experiments designed to test them.
Background

Escherichia coli bacteria were consistently killed when treated in the flat-plate apparatus seen in Figure 1. When bacteria were suspended in agar, sandwiched between the two metal plates, and shocked for 90 minutes there was significant killing. However, when bacteria were treated in a liquid buffer solution there was no killing. However, since agar is a gelatin-type material at room temperature and the buffer is liquid they had to be treated in different apparatus. The liquids were all electrically treated in the vertical-tube apparatus seen in Figure 2. Comparing the two figures quickly demonstrates several key differences between the two apparatus; the thickness of the bacterial suspension varied while the current/cm2 was kept the same.

Experiments
In order to test the agar media in the vertical tube apparatus we needed to create a method for removing a known volume of media before and after testing. While it may seem like a simple problem we struggled for quite some time to devise a method that was both simple and extremely accurate. Even the slightest inaccuracy in the volume removed would result in very inaccurate results due to the size of bacteria. We decided that weighing each sample of agar on a balance was the simplest method, but in order to calculate the amount of bacteria killed we needed to know the volume of the sample, not the mass. In order to convert from mass to volume we needed to know the density of the sample. This proved to be the most difficult process of my entire project. Density is calculated by measuring the volume of the object, the mass of the object, then dividing the mass by the volume. However, our entire purpose in having the density was to calculate the volume! We investigated several methods of calculating density for several weeks before inventing one that proved successful. I created a salt solution of medium density and placed a small piece of agar into the solution, being especially careful not to trap any air bubbles beneath the agar. If the agar was less dense then the solution it would float; more dense—sink; and equally dense—remain suspended in the solution. More salt was added step by step to slowly increase the density of the solution. The entire process was repeated multiple times over several days because our initial densities were much too low. Eventually the correct density solution was created and the density of our agar samples determined.
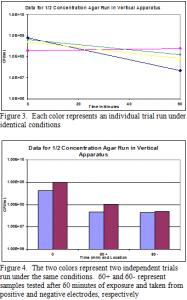
The first tests conducted used agar as a suspension media in the vertical tube apparatus. Agar samples were removed before and after treatment, weighed on a balance and diluted. The diluted bacteria were then counted. Figure 3 shows the results obtained. With one exception, they do show a decrease in bacterial growth after treatment, but nowhere near the decreases previously seen in the flat plate apparatus. In order to investigate variation due to the increased thickness of the sample we attempted to test bacterial killing at different distances from the positive electrode. The suspensions were tested in the vertical tube apparatus, removed and frozen. Once frozen, we were able to remove and dilute slices from the positive end, the center of the core, and the negative end. However, overnight growth showed the freezing procedure itself had killed all the bacteria (data not shown). We decided to test the same variable with a different procedure, this time simply scooping out a sample of agar from the positive and negative electrode sides of the sample. Unfortunately, this method does not allow for a sample equidistant from both electrodes. These results can be seen in Figure 4. These data show a decrease in growth comparable with that seen in Figure 3. There is no significant difference between the samples taken at the positive and negative electrodes.
Conclusions
These data represent an increased understanding of this phenomenon; nevertheless, many aspects require additional investigation. The geometry of the apparatus does affect bacterial killing, but it is clearly not the sole variable. The proximity to the positive or negative electrodes also appears to have little effect on killing. One additional variable that went untested was the ion concentration of the agar solutions versus the liquid suspension.
While these experiments do not provide a complete understanding of electricity as a bactericidal agent they do provide additional background on which to build. In addition the experience gained throughout this process has proven invaluable to my progress as a scientist. Currently I am finishing my first semester as a graduate student. As in the project described here I am constantly required to overcome obstacles and failures with creativity and ingenuity. I believe it is a process that all researchers go through and must learn to deal with. At times it can be very frustrating, but having gone through a similar process and eventually produced results is an experience that helps me to keep looking for solutions.
