Chad Varner and Dr. Brad Bundy, Department of Chemical Engineering
The purpose of this project has been to help develop a novel nano-imaging particle. Current methodologies have limitations that preclude them from being used for nanometric (1-500nm) imaging processes in living organisms. These include, but are not limited to, low signal to noise ratios, limited target specificity, and bio-incompatibility. To further the possibilities available for nano-imaging, we have attempted to create a scaffold, or framework, for a biocompatible nanoparticle that is capable of imaging specific targets in living systems.
To do this, we hypothesized that the QB virus’s protein shell could be modified for imaging applications. Viral nanoparticles (VNPs) have been shown in the past to be adept for imaging when they are covered with fluorophores1. However, because most VNPs are composed of many copies of a few proteins, finding a way to attach the particle to specific targets is difficult. This targeting ability is what makes the Qb VNP special, and it is the attribute we aimed to exploit while undertaking this research. In its natural state, the QB VNP includes one copy of a maturation protein, called the A2 protein, into its shell2. Our hypothesis was that if we could modify the A2 protein such that it exposed a targeting residue to the outside solution, we could use it as an attachment site while the rest of the VNP would be covered with fluorphores for imaging. A cartooned diagram of what this particle would look like is shown in Figure 1 with the targeting residue on the A2 protein shown as a black star and the fluorophores depicted in orange.
To confirm our hypothesis, we had four main objectives. They were to 1) identify sites on the A2 protein that would likely be surface accessible, 2) incorporate the targeting molecule into the A2 protein at these various points, 3) produce a QB VNP with a modified A2 protein included, and 4) show that the targeting molecule was still accessible to be bound to a target. The remainder of this report is dedicated to explaining the efforts and results pertaining to these objectives.
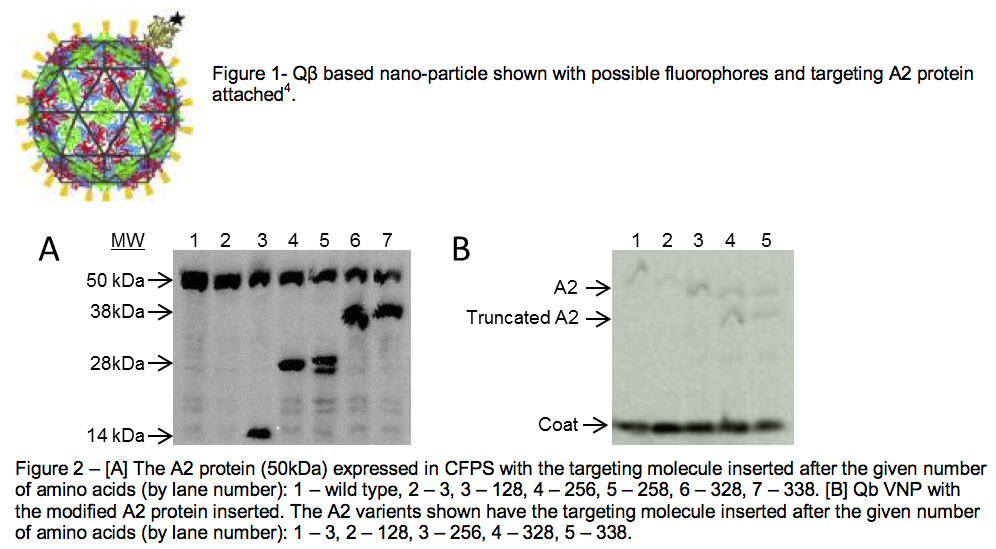
Identifying sites on the A2 protein that could be modified posed the first challenge. Because the A2 protein is cytotoxic and very insoluble, the structure of the protein has never been solved. Also, no one knows which sections of the A2 protein are directed inward or outward when it is incorporated into the QB VNP. We chose to use a protein modeling software, I-TASSER, to estimate certain regions of the A2 protein that would likely be surface accessible3. In fact, the image of the A2 protein shown in Figure 1 was generated using this program. Since the overall orientation of the A2 protein was unknown, we inserted our targeting molecule at 6 different sites throughout the protein. We then used standard DNA cloning techniques to make the new DNA, and a cell-free protein expression system (CFPS) to produce the mutated proteins (see Figure 2A)4. The dark bands at the lengths of the 6 different insertion sites show that the protein sometimes is truncated rather than proceeding to create the full-length product.
Our third objective was to incorporate these modified A2 proteins into QB VNPs. To isolate those A2 proteins that were included in the VNP from A2 proteins that were free in solution we separated the complete VNPs from any unincorporated proteins using an ultracentrifuge that subjected our reactions to forces 105,000 times gravity. The difference in density of the VNPs and free proteins was exploited and the VNPs were purified from all other proteins. After we had purified VNPs, we showed that they in fact did contain the A2 protein by using a protein gel. Figure 2B is an image of our VNPs, with the proteins separated by size. The image clearly shows that the A2 protein was included in the VNP. It also shows that A2 protein does not need to be its full length to incorporate into the VNP (see lanes 4 and 5).
The completion of our final objective is currently underway. To verify finally that the A2 protein is still available to be bound to a target when it is inserted into the VNP, we are binding the targeting molecule in the A2 protein to 2 different targets using “click” chemistry. We are using magnetic beads and long hydrocarbon chains. Both of these attachment reactions are being done when the VNP is still intact. Currently, we are using radiation measurements and autoradiography to assess attachment to the magnetic beads and hydrocarbons, respectively. If the A2 protein successfully binds to either of these targets then the novel VNPs that we have created are in fact viable to be used in targeting applications. These results will soon be submitted to a peer-reviewed journal such as Biotechnology Progress5.
References
- Ewers, H.; Smith, A. E.; Sbalzarini, I. F.; Lilie, H.; Koumoutsakos, P.; Helenius, A., Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc Natl Acad Sci USA 2005, 102, (42), 15110-5.
- Bernhardt, T. G.; Wang, I. N.; Struck, D. K.; Young, R., A protein antibiotic in the phage Qβeta virion: diversity in lysis targets. Science 2001, 292, (5525), 2326-9.
- Yang Zhang. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 2008, vol 9, 40.
- Varner, C.T.; Smith, M.S.; Bundy, B.C., Optimization of A2 incorporation into the Qβ Virus-Like Particle Enabled by an Open transcription/Translation Environment. Biotech Prog 2011, 28, 549
- This work was funded in part by BYU ORCA. The author also acknowledges the help of Mark Smith, a graduate student working in Dr. Bundy’s lab.