Adam Broderick and Dr. Ken Solen, Chemical Engineering
Hypothermia-Induced Platelet Aggregation (HIPA) may pose a significant risk for patients undergoing some surgical procedures. During heart surgery, a patient’s core temperature is typically lowered to reduce tissue damage. Hall et al. (2002) observed that this caused blood platelet aggregation in ~30% of test subjects1 and increases post-operative neurological impairment after hypothermic coronary bypass surgery, possibly through occlusion of small blood vessels by platelet aggregates.2
Hall et al. discovered that fibrinogen (a protein essential to blood clotting and aggregation) from the blood of those exhibiting HIPA precipitated under different conditions than fibrinogen of those not exhibiting HIPA.1 No other information is available to prove or disprove a possible correlation between fibrinogen functionality and HIPA. The purpose of this project was to provide data either proving or disproving the hypothesis that people exhibiting HIPA have abnormal fibrinogen, and with additional research could lead to a method of identifying and treating HIPA.
Fibrinogen, like all proteins, has a functionality that is dependent on its concentration and chemical structure. Differences in chemical structure can affect how it interacts with other chemicals and proteins, causing the molecules to bind more tightly, while concentration affects the likelihood of molecular interactions. We hypothesized that one of these two factors could be important in explaining the connections between HIPA and fibrinogen.
The first step in the process was indentifing human subjects that fell into the catagories of high, medium, and low responders. While I helped occaisionally with finding subjects and collecting data, this was not the focus of my research project and thus only the results of the screeing are included in this report.
Second, the functionality of fibrinogen was measured using two tests: clotting time and clot adhesion strength. In the first, the fibrinogen solution was mixed with a protein that causes the fibrinogen to clot, much like the natural immune response in the case of bleeding. Higher concentrations of fibrinogen cause the solution to thicken faster, and thus the time required to reach a certain viscosity can be correlated to concentration. Comparison to a standard curve gives an approximate concentration. In the second test, the same protein was used to clot the fibrinogen, but the clot was formed between two small pieces of bovine vascular tissue. The clot adheres to the tissue, and a tensile machine was used to measure the force required to pull the pieces apart.
Fibrinogen concentrations and tensile strength data were collected from 15 human subjects, 10 of which were low responders and the remaining 5 high responders. The number of high responders was limited by the screening process, as many subjects had to be screened to get a few high responders. Figures 1 and 2 show the results from each test.
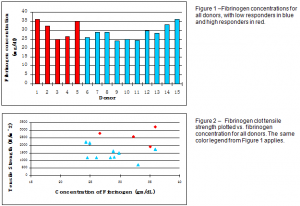
Fibrinogen concentrations were in the same range for both high and low responders, with similar high and low concentrations, indicating no statistical difference in fibrinogen concentrations. There is, however, a separation between high and low responders in the graph of tensile strength vs. fibrinogen concentration. This was verified statistically with a two-tailed t-test (p = 0.0036).
It can be concluded, therefore, that there may be some difference in the structure and functionality of fibrinogen in high and low responders which can cause HIPA. The higher clot tensile strength of the high responders is consistent with the hypothesis of heightened ‘stickiness’ of fibrinogen which causes the platelets to aggregate. Further experiments might include more comprehensive structure analysis and sequencing to determine what structural differences exist between fibrinogen in high and low responders.
Sources
- Hall, Matthew, et al. “Hypothermia-induced platelet aggregation in heparinized flowing human blood: Identification of a high responder subpopulation.” Am J Hematol. 69 (2002):45-55.
- Hall, Matthew, et al. “Hypothermia-induced platelet aggregation and cognitive decline in coronary artery bypass surgery: A pilot study.” Perfusion. Submitted for publication.
