Robert A Burton and Dr. Noel L. Owen, Chemistry and Biochemistry
The seed of the Futu tree, Barringtonia asiatica, has been used by inhabitants of the Pacific islands for many years as a highly toxic fish poison.1 These ethnobotanical studies were responsible for the initiation of this investigation.
Successful completion of this project required a specific bioassay to determine toxicity. Initially the toxicity of the compound was determined using a standard MTT cancer cell line bioassay.2 However, the compound in question was not very cytotoxic and therefore a feeder guppy bioassay was developed. This new bioassay worked extremely well. Impure fractions containing the compound responsible for the biological activity was toxic to the feeder guppies at a concentration of 500 ng/mL. Further tests are currently being conducted to determine the exact toxicity of the purified compound.
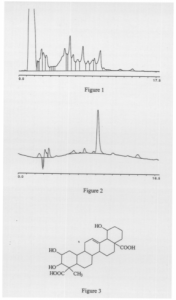
Isolation of the bioactive components was performed through liquid/liquid separation and HPLC (High Performance Liquid Chromatography). After each step of the separation, the feeder guppy bioassay was used to identify which fraction contained the active compound. These studies resulted in an optimized isolation scheme for the active component. The seed was first ground and partitioned between water and butanol. The butanol fraction, which contained the active component, was then dried to a solid and partitioned between ethyl acetate and acidic water (acidified with 0.l% trifluoroacetic acid). Any insoluble material was subjected to centrifugation and added to the water fraction. The solvent was removed from the combined water/precipitate and resolubilized in water (pH 4) and injected onto the HPLC column. Final purification of the compound was performed on preparative HPLC with an isocratic gradient of 50/50 water/acetonitrile with 0.1% trifluoroacetic acid (Fig. 1,2).
With the purified compound, spectral studies have begun in an effort to determine the chemical structure. Preliminary NMR spectra have been obtained and future experiments include: 1H, 13C, COSY, DEPT, and HETCOR. Mass spectral data indicates a formula weight greater than 400. A high resolution MS will be obtained on a highly purified sample. From the spectral data obtained thus far, it is believed that the active compound is a triterpene acid. Triterpenoids have been isolated previously from Barringtonia species (Fig. 3).3 Therefore, these compounds will be compared initially with the characteristics of the isolated fish poison to determine whether they are similar in structure.
References
- Cox, P.A. J of Economic Botany 1979, 33, 397-99.
- Hawcroft, D.M. Quantitative Bioassay. 1987 Chichester New York, NY.
- Subba Rao, G.S.R. Phytochemistry, 1981, 20, 333-34.
- The aid of Dr. Steven G. Wood and Dr. Du Li of the Department of Chemistry and
Biochemistry is gratefully acknowledged.
FIG. 1 HPLC chromatogram of crude butanol extract.
FIG. 2 HPLC chromatogram of purified active component.
FIG. 2 Structure of bartogenic acid, a triterpene isolated from Barringtonia asiatica.