Sara Ehlert and Dr. Aaron Hawkins, Department of Electrical Engineering
The importance of blood to humans cannot be understated. Its important functions include transporting oxygen and nutrients around the body, preventing blood loss, fighting infection, carrying antibodies to fight infection, delivering waste products to the kidneys and liver to be removed from the body, and regulating body temperature [1]. Unfortunately, there are several severe diseases that are directly related to blood, including anemia, hemophilia, blood cancers, blood clotting problems, septicemia (blood poisoning), and sepsis. Furthermore, many important diagnostic tests are performed using blood, such as evaluating organs (e.g. kidney, liver, thyroid, and heart), diagnosing cancer, HIV/AIDS, diabetes, and medicine effectiveness [2]. Blood filtering is critical to both diagnosing and treating these conditions.
Generally speaking, when diagnosing diseases or medical conditions, only a small sample of blood is needed. However, some tests take over a day to get results, often because the lab work is outsourced to a specialized off-site facility. The lab-on-a-chip research field aims to put fast, low-cost diagnostics at the point of care. Currently, there isn’t a very efficient method of filtering whole blood into the constituents needed for testing on a single chip. Furthermore, treatments for diseases involving blood often require removing undesirable cells in the blood. For example, in dialysis, waste products must be removed from the blood. Additionally, treating sepsis, a disease where the body has a severe response to bacteria or other germs [3], could include filtering the blood to remove harmful bacteria or viruses before returning the blood back into the body.
Because of the evident need of a more efficient way to filter blood in both large and small scale applications, my research aims for an extracorporeal system for treating blood without the need for anticoagulants. This is vital, because those who have suffered a traumatic experience often suffer from coagulopathy, due to blood loss, hypothermia, and acidosis [4,5]. Furthermore, by not requiring the use of anticoagulants, such a filter can be implemented in remote locations where anticoagulant therapy may be unavailable. In particular, a microfilter we are developing will be used as a first filtration pass to separate red and white blood cells from plasma, bacteria, and viruses. The microfilter will then be integrated into a larger system that further separates the blood to discard harmful bacteria, viruses, cytokines, and toxins before returning to the patient, thus providing the body with assistance in removing harmful particles from the bloodstream.
Filters constructed using microfabrication techniques have important advantages, including high scalability, low cost manufacturing, extreme precision, and relative ease of integration with other lab-on-a-chip features.
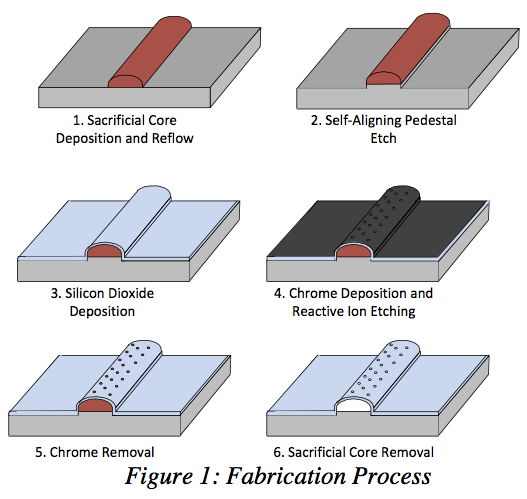
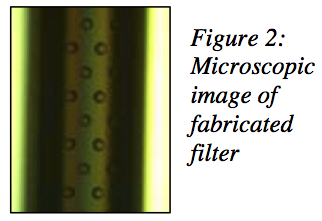
We were able to fabricate high-strength glass capillary structures with arched profiles are constructed on silicon wafers in lengths up to 10 cm. Resulting wall thicknesses are approximately 5 μm, and filtration holes are etched with high density and dimensional accuracy using reactive ion etching (RIE) in the BYU cleanroom facility. As many as 4.6 million equally spaced 5 μm diameter holes can be etched into a 1 cm2 area, and parallel arrays of microchannels can be coCnrsotrsusc-tFeldotwo aFcicltormatmiondate high fluid flow rates (see Figures 1 and 2).
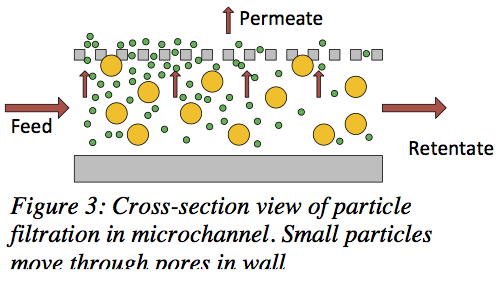
Our microfilters are specifically designed to capitalize on the benefits of cross-flow filtration, a type of filtering in which the bulk of a fluid moves tangentially to the pore membrane (see Figure 3). The benefits of cross-flow filtration over traditional, dead-end filtration include a prevention of filter cake formation and continual fluid flow and filtering action.
Currently, we are testing our microfilters using polymer beads with fluorescent labels. In order to test the separation efficiency of the microfilters, we are using the beads to observe and quantify the efficiency of the filtration and fluid flow rates. We will then move on to testing with blood products through collaborations with the Chemical Engineering and Biochemistry Departments at BYU.
In conclusion, the need to develop efficient filters for blood testing or point of care treatment is an urgent, unmet need. Dr. Hawkins and I have been working on fabricating a cross- flow filtration system using techniques from the microfabrication industry and microfludic research to meet this need. A poster discussing this research is displayed on the south side of the 4th floor of the Clyde Building.
References
[1] http://www.hematology.org/Patients/Blood-Basics/5222.aspx
[2] http://www.nhlbi.nih.gov/health/health-topics/topics/bdt/
[3] http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001687/