Bryant Staples and Dr. William Pitt, Chemical Engineering
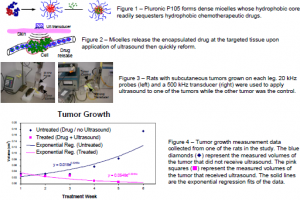
The tri-block copolymer, Pluronic P105, has been found to be an ideal ultrasonically activated drug delivery vehicle because it forms micelles with hydrophobic polypropylene oxide cores that sequester hydrophobic drugs (Fig. 1). These micelles release their contents upon the application of low frequency ultrasound [1] such that drugs can be released specifically at the ultrasonicated region (Fig. 2). Such ultrasonically controlled release has been effective against cancer cells in vitro [2] and in vivo [3].
The purpose of this research is to assess the use of these novel polymeric micelles in ultrasonically-activated Doxorubicin® (DOX) delivery to tumors. This cancer therapy involves the exposure of the animal to localized ultrasound. Currently, one of the most effective therapies for cancer treatment involves the use of chemotherapeutic agents such as DOX. One of the major drawbacks of this therapy is that the drug attacks all rapidly dividing cells, causing healthy tissues to die. This localized treatment using micelles and ultrasound may alleviate the negative side effects of the drug on healthy tissues. Encapsulation prevents drug interaction with cells. Localized release of DOX by ultrasound limits the areas within the patient where the drug can take effect. This project’s goal is to determine if tumors exposed to ultrasound after receiving DOX loaded micelles show a smaller growth-rate compared to tumors with the same DOX-loaded micelles that do not receive ultrasound. Also studies were performed to determine the concentration verses time difference (if any) between ultrasonicated and non-ultrasonicated tumors; and fluorescent microscope pictures were taken for comparison.
Methods
The drug carrying micelles were formed from PluronicTM P105, a tri-block copolymer consisting of a central block of poly(propylene oxide) flanked by blocks of poly(ethylene oxide). These micelles were stabilized by polymerizing an interpenetrating network of a thermally responsive N,N-dimethylacrylamide within the core of the micelle [4].
DHD/K12/TRb rat colonic cancer cells were subcutaneously injected and grown in each lower leg of the BIDX rat model. All rats received an injection of micellar-encapsulated DOX at 2.67 mg/kg. Ultrasound exposure followed for a period of fifteen minutes to only one leg of the animal. Ultrasound was applied by either a 20 kHz probe or a 500 kHz transducer (both at 1.0 W/cm2) in ultrasound-conducting gel on the skin over the tumor (Figure 3). Treatments were given weekly for six consecutive weeks, during which the tumor sizes were measured. Each rat was euthanized at 0.5, 3, 6, or 12 hours after ultrasound application.
After euthanasia, DOX was immediately extracted from the tumors (both the ultrasonically treated and untreated) and quantified using high-performance liquid chromatography and a fluorescence detector.
Results
Volume measurement of tumor growth data fit best to an exponential regression : V(t) = Aekt
(See Figure 4 below). Comparison of the exponential constants (k) between the ultrasonicated tumors and non-ultrasonicated tumors showed that the ultrasonicated tumors displayed slower growth (P < 0.0047). Comparison between tumors which received 20 kHz ultrasound, 500 kHz ultrasound, and no ultrasound showed no statistical difference in drug concentration within the time interval studied. The drug concentration in the tumor remained steady between 0.8-1.0 mg Dox / g tumor within the time interval studied.
Conclusions
Ultrasound application reduced the tumor growth rate compared to the non-ultrasonicated tumor within the same rat. However, the Dox concentration in the tissue appears independent of ultrasound application. This rejects the hypothesis that the decreased tumor growth rate is because the ultrasound treatment locally increases the drug concentration.
References
- Husseini GA, et al. Colloids and Surfaces B-Biointerafces, 2002; 24 (3-4): 253-264
- Husseini GA. Cancer Lett 2000; 154:211-6
- Nelson JL., et al. Cancer Reasearch, 2002 62: p7280-7283
- Pruitt JD. Macromolecules 2000; 33 (25): 9306-9