Douglas S. Lewis and Dr. William Pitt, Department of Chemical Engineering
Introduction
In the past decade, many efforts have been made to improve chemotherapeutic treatments for cancer patients. One of the specific efforts seeks to actively deliver anti-cancer drugs to the target tissue rendering the chemotherapy more efficient and less damaging to nearby uninfected cells. The lab of Dr. William Pitt is currently investigating the site-directed delivery of drugs using polymeric nanocarriers that release their contents upon irradiation with ultrasound (US).1-4 In a typical application, without active targeting, drug sequestered in a carrier is infused into the circulatory system and the US is focused on the tumor site, such that as the polymeric carriers flow through the insonated volume, they release their contents to the tumor tissue. The downside to this current approach is only 10% of the drug content is released per insonation event. This observation led to the hypothesis that if it were possible to localize all of the polymeric carriers in the insonated volume, the efficiency of drug release could be greatly improved through multiple insonation events. Thus, this report presents the work I have done to synthesize a targeting drug delivery carrier by conjugating antibody (Fab’) fragments to poly(ethylene glycol) (PEG) chains on the surface of the carrier.
Methods
The synthesis of the antibody-conjugated PEG can be divided into two phases: 1) PEG Activation and 2) Fab’ Preparation and Attachment. A detailed description of each phase is included as an appendix to this report. A summary of this procedure follows below.
PEG Activation – Dry monomethoxy-PEG (5,000 MW) was dissolved in dry dichloromethane along with excess acryloyl chloride and triethylamine and stirred under nitrogen overnight at 25°C. The resulting PEG-acrylate was dried with anhydrous MgSO4, precipitated in ether and characterized by H-NMR, and stored at -20°C.5
Fab’ Preparation and Attachment – Sheep IgG was purchased from Sigma. The F(ab’)2 fragments were prepared using an ImmunoPure F(ab’)2 Preparation Kit (Pierce, Rockford, IL). The fragments were dialyzed and then reduced with 2-mercaptoethylamine as described in a previous paper.6 Reduced Fab’ fragments were immediately added to a PEG-acrylate solution with stirring for 12 hours at 25°C. Unreacted PEG was separated from the PEG-Fab’ using a Sephadex-G25 column. Characterization of the final product was accomplished through TLC and H-NMR analysis.
Results and Discussion
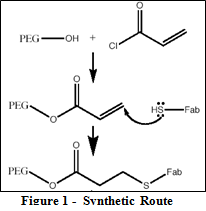
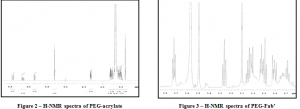
The synthetic route described previously is illustrated in Figure 1. As evident from the diagram, the synthesis takes advantage of a relatively simple condensation reaction between the –OH end and the reactive acryloyl chloride thereby producing a reactive vinyl cap on the PEG chains. Figure 2 shows the H-NMR spectra of the PEG-acrylate product with the PEG hydrogens located at 3.6 ppm and the vinyl hydrogens at the 3 characteristic peaks between 5.8 and 6.5 ppm. Integration of these peaks proved that approximately 60% of the PEG chains were capped with acrylate ends. The sulfhydryls on the reduced Fab’ fragments then react with the vinyl caps to accomplish the covalent attachment of the targeting moiety. This particular synthesis led to 50% yield. Figure 3 shows the H-NMR spectra for the final PEG-Fab’ product. This spectra contains the characteristic PEG peak at 3.6 ppm and an abundance of protein peaks from 2.6 – 3.4 ppm. Having these proteins peaks on the spectra after passing the final product through the Sephadex-G25 column confirms that the Fab’ fragments are indeed covalently attached and are not just separate peptide fragments in solution.
Conclusion
This synthetic pathway is useful in attaching antibody fragments to PEG chains on the surface of a micellar drug carrier. In vitro and in vivo tests should be performed to confirm the hypothesis that the efficiency of drug release will increase by directing the drug carriers to the target location. I am currently working towards progress in this direction.
References
- Zeng, Y et al. J Biomat Sci Polym Edn 17, 591-604 (2006).
- Pruitt, JD et al. Macromolecules 33, 9306-9 (2000).
- Husseini, GA et al. J Control Rel 69, 43-52 (2000).
- Husseini, GA et al. J Control Rel 107, 253-61 (2005).
- Hahn, MS et al. Biomaterials 27, 2519-24 (2006).
- Jeong, JH et al. J Control Rel 107, 562-70 (2005).
APPENDIX
Preparation of acrylated PEG
1. Dissolve .1 mmol of –OH capped PEG in 10 mL of anhydrous dichloromethane (DCM).
2. Add .2 mmol triethylamine and 1 mmol acryloyl chloride (10-fold excess) to the solution.
3. Stir overnight under an inert gas (N2 or argon).
4. Wash the resulting solution with 2 M K2CO3¬ and separate into aqueous and DCM phases to remove the HCl.
5. Dry the DCM phase with enough anhydrous MgSO4 until the MgSO4 is free-flowing.
6. Precipitate the acrylated-PEG in 100 mL of diethyl ether.
7. Centrifuge the ether/DCM solution at _ for at least 5 min (all of the solid should settle at the bottom)
8. Decant off the solution and redissolve in 5 mL of DCM.
9. Transfer solution to a small vial and evaporate off the solvent with a vacuum pump. Record the mass of the recovered PEG.
10. Use immediately or store in freezer . A small sample of this product can be dissolved in CDCL3¬ w/ TMS (3wt%) for NMR characterization.
Preparation of Fab’ fragments
Digestion Buffer – Dissolve 2.72 g of sodium acetate, trihydrate in 1 L of ultrapure water to prepare 20 mM solution. Adjust pH to 4.5 and store at 4°C.
Preparation
1. Buy ImmunoPure F(ab’)2 Preparation kit (Pierce)
2. Equilibrate AffinityPak Protein A Columns and ImmunoPure Buffers to room temperature.
3. Using a cut pipette tip, place .25 mL of the 50% immobilized pepsin slurry into a 16x150mm glass test tube and add 4 mL of Digestion Buffer.
4. Separate immobilized pepsin slurry from the buffer using the resin separator. The resin separator is inserted into the tube slightly compressing the gel. The buffer can then be decanted (See kit instructions for diagram of how to use the resin separator). Repeat this wash procedure. Resuspend the immobilized pepsin in .5 mL of Digestion Buffer.
5. Dissolve 10 mg of salt-free, lyophilized IgG in 1.0 mL of Digestion Buffer.
Fragment Generation
1. Add 1.0 mL of the dissolved IgG to the tube containing the equilibrated immobilized pepsin (.5 mL). Position the resin separator inside the tube just above the reaction mixture.
2. Incubate IgG for 4 hours in a shaking water bath at 37°C at high speed. Maintain constant mixing of gel during incubation.
3. Separate digest from the immobilized pepsin using a resin separator. Decant the crude digest with F(ab’)2 fragments into a new test tube.
4. To maximize the fragment recovery, wash the immobilized pepsin with 1.5 mL of ImmunoPure IgG Binding Buffer. Add the wash to the crude digest. The total sample volume should be 3.0 mL. Discard the used immobilize pepsin.
F(ab’)2 Purification
1. To avoid air bubbles being drawn into gel, open an Immobilized Protein A Column by removing the top cap first. Discard the storage solution (contains 0.02% sodium azide).
2. Remove bottom cap from the column. Equilibrate column by adding 12 ml of Binding Buffer and allowing the solution to drain through.
3. Transfer column to a 16×150 mm test tube labeled F(ab´)2.
4. Apply the crude digest (3.0 ml) to the column and allow it to flow completely into the gel. Column will stop flowing when the liquid level reaches the top frit. Add 7 additional mL of Digestion Buffer to allow all of the F(ab’)2 to come out of the column
5. Collect 1 mL fractions and perform UV-spectrocospy on each to determine protein content
6. Pool fractions with significant protein concentration together
7. Perform dialysis with 50K MWCO membranes to remove unwanted Fc fragments. Dialyze the F(ab’)2 solution against the Reduction Buffer three times for adequate buffer exchange.
F(ab’)2 Reduction/ Fab’ Generation
1. Prepare a 100 mL sample of Reduction Buffer with 6.6 g (66mg/mL) of 2-mercaptoethylamine.
2. Add F(ab’)2 solution to 2-mercaptoethylamine reduction buffer in a 10:1 volume ratio (2-mercaptoethylamine will be diluted to 6mg/mL).
3. Place the resulting F(ab’)2 solution in shaking water bath at 37°C for 90 minutes.
4. Remove the excess 2-mercapto ethylamine by applying solution to a Sephadex-G25 column.
5. Collect 1 mL fractions and monitor protein content with UV-spectroscopy.
6. Pool fractions with significant protein concentration together.
Antibody Attachment
1. Add resulting Fab’ solution to vial containing the acrylated PEG dissolved in 5 mL of reaction buffer. Add sufficient 1 M HEPES solution to bring HEPES concentration to 10mM.
2. Allow the reaction mixture to mix for 12 hours at room temperature.
3. Remove unreacted polymer chains by applying the solution to a Sephadex-G25 column.
4. Collect 1 mL fractions and monitor protein content with UV-spectroscopy
5. Concentrate product by evaporating off some of the water with a vacuum pump. Final product can be characterized with TLC and NMR.