James Lattin and Dr. William Pitt, Chemical Engineering
For over a decade ultrasound enhanced drug delivery systems have been under development. These systems often use polymeric molecules that form micelles. In aqueous solutions (such as blood), a spherical micelle is formed as the hydrophilic ends of the molecules interact with the solution and the hydrophobic ends are pushed to the inside of the sphere where they can interact with each other. Hydrophobic molecules, such as the anticancer drugs or gene vectors can be sequestered inside of the micelle. At the desired location, the micelle is broken apart with ultrasound and the drug is released. This allows for localized treatment without exposing the entire body to the drug. In the example of cancer treatment, the same drugs could be used as in normal chemotherapy, but without the widespread side effects. One downfall to some of the currently studied drug delivery systems is that they leave non-biodegradable waste products in the bloodstream that can cause kidney damage. Our objective is to develop a suitable drug carrier for ultrasound enhanced treatment that would be biodegradable. We are working towards synthesizing a tri-block polymer with Poly-ethylene oxide (PEO), Poly-propylene oxide (PPO), and Poly-lactic acid (PLA) blocks. The PEO and PPO blocks will provide the hydrophilic end of the molecule and the PLA block will add the biodegradable hydrophobic end.
We obtained a PEO750-PPO600 di-block copolmer with a hydroxyl group on the PPO end from Advanced Polymer Materials, Inc. Because this polymer was expensive, we began experiments with Pluronic F127 (BASF Corp.), a PEO-PPO-PEO tri-block copolymer with hydroxyl end groups. Using a hot oil bath, a mixture with a 2 to 1 weight ratio of Lactide monomer to F127 is melted at approximately 130 °C. Stannous heptanoate (0.5% wt of lactide) is used as a catalyst. To facilitate its addition to the melted mixture, the catalyst is dissolved in toluene. The mixture is then heated for one day under nitrogen at temperatures between 140 °C and 180 °C. The F127-PLA copolymer is precipitated from methanol to separate it from unreacted F127 and lactide monomer.
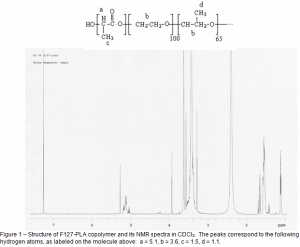
I am using H-NMR and HPLC to characterize the products. Figure 1 shows an NMR spectrum of one of the obtained products. NMR spectra have shown that PLA is forming on the end of the existing PEO blocks. However, some experiments have yielded either very messy spectra (like the one shown in Figure 1) or have barely shown the important peaks. The first case suggests that the F127-PLA product has not been sufficiently isolated, and the second case suggests that very few lactide monomers were added to the chain.
While the experiments have shown some promise, there are a number of difficulties that continue to be addressed. Perhaps the largest difficulty has been that all reaction conditions tried have resulted in an extremely low yield. While sufficient F127-PLA product is created to characterize by NMR or HPLC, it is not enough to test its ability to sequester DOX. Furthermore, the low yield makes it unreasonable to try the reaction with the more expensive PEO750-PPO600 di-block copolymer.
I have found that higher temperatures (155-180 C) are necessary to form a substantial PLA block, but the molten mixture turns brown after several hours, suggesting that oxygen is being allowed into the reaction flask and some of the reactants oxidize at these higher temperatures. While lower temperatures do not produce this brown color, the reaction proceeds too slowly to form a substantial PLA block.
I have also found that great care must be taken to expel any water. Best results have been obtained when the reactants are dried in a vacuum oven and glassware is flame dried immediately prior to starting the polymerization. Keeping water and oxygen out for the duration of the experiment is especially difficult because the reaction is very slow.
Future work will include running the reaction longer and at higher temperatures with a stronger nitrogen purge. I will continue to work to improve the yield, and experiments will also be done with alternate solvent systems for improved product recovery. When yield and purity of the product has been increased, the experiment will be repeated using the PEO750-PPO600 di-block copolymer. Lastly, experiments will be done to test the ability of the resulting micelle to sequester drugs and release them when exposed to ultrasound.
