Robert Blake and Dr. Matthew R. Linford, Chemistry and Biochemistry
Silicon is one of the most abundant elements on the earth and has been used throughout history in a variety of applications. Because silicon is so widely used in the semiconductor and electronics industries, recent research has focused on altering the properties of silicon wafers. Chemical modification of the silicon surface changes the properties of the silicon wafer and potentially presents new semiconductor applications. One method commonly used to modify silicon surfaces is to grow polymer brushes from them.
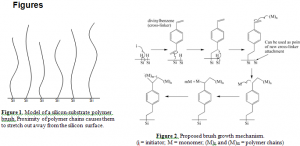
Polymer brushes are composed of polymer chains, large molecules made of repeating subunits called monomers, tethered to a surface or substrate. The polymers are attached at such a high density that the chains stretch away from the surface to avoid overlapping (Figure 1). Because of their unique configuration, polymer brushes alter the properties of the surfaces to which they are bound.1
The hydrogen-terminated silicon surface is commonly used for making polymer brushes. Hydrogen-terminated silicon is particularly useful because the Si-H bond has a relatively weak bond strength and can easily be broken. This makes the Si-H bond a susceptible site where other atoms can bind to the silicon surface.2
Polymer growth on hydrogen-terminated silicon surfaces is usually multi-stepped, difficult to carry out, time consuming and expensive. We created a new method that involves a single-step process by which polymer brushes can be simultaneously grown and attached to hydrogen-terminated silicon surfaces. This is done by preparing a solution containing all the chemicals necessary for the polymerization reaction. These include free radical initiators (molecules that create a free radical or unpaired electron), reactive monomer molecules and cross-linker molecules (monomers that have two reactive ends). A hydrogen-terminated silicon wafer is placed in this solution, and a polymerization reaction is carried out. Free radicals react with the Si-H bond to create surface-bound silicon radicals, which can then react with one end of the cross-linker molecules, forming Si-C bonds. Growing polymer chains in solution then react with the other end of the cross-linker molecules, effectively binding them to the surface (Figure 2).
The first sets of experiments we did were with the monomer methyl acrylate and the cross-linker divinylbenzene. By measuring the thickness of the polymer film using optical ellipsometry, we found that we could grow thick polymer films using our approach, between 8-15 nanometers (nm). Both the monomer and the cross-linker molecules are necessary, because if a solution is made that contains only the monomer or the cross-linker, but not both, the thickness of the film is comparatively very small. Additionally, we found that the film thickness of the polymer brush was proportional to the concentration of the divinylbenzene that we used.
In the next set of experiments we used different monomers with the same cross-linker as before, divinylbenzene. We tried our experiments with the monomer methyl methacrylate and were able to obtain film thicknesses of 10-16 nm. We repeated the experiments with the monomer styrene and again found we could grow polymer films of significant thickness, between 10-13 nm. This indicates to us that the same procedure could be used with any variety of monomers, allowing scientists and companies to adjust the properties of silicon surfaces to fit their needs.
Finally, we did experiments with another cross-linker, 1,3 butanediol diacrylate. Using this cross-linker with the monomer methyl methacrylate, we made polymer films that we 6-8 nm thick on a consistent basis, and even made a few that were much thicker. The ability to use more than one cross-linker is significant because it shows that the procedure is not bound by having to use the same molecule every time. Different cross-linkers can be used, which can change the properties of the final polymer film and silicon surface.
By introducing this simple, one-step method for growing polymer chains on hydrogen-terminated silicon surfaces we hope to allow for a cheaper, easier way to covalently modify silicon wafers. This will help further advances in polymer and surface chemistry research and in the semiconductor industry.3
References
- Yu, W. H.; Kang, E. T.; Neoh, K. G. Langmuir 2004, 20, 8294–8300.
- Sun, Q.; de Smet, L. C. P. M.; van Lagen, B.; Giesbers, M.; Thüne, P. C.; van Engelenburg, J.; de Wolf, F. A.; Zuilhof, H.; Sudhölter, E. J. R. J. Am. Chem. Soc. 2005, 127, 2514–2523.
- The following also contributed to the research: Michael V. Lee, Lei Pei, Naoto Shirahata.