Stuart Thomas and Dr. Scott Steffensen, Neuroscience Center
Each year, more and more people become the slaves of addiction to narcotics, alcohol, and nicotine. These addictions destroy families and ruin lives. The brain’s chemistry is influenced by these drugs. This study aimed to evaluate how alcohol and other drugs affect the brain’s chemistry and identify potential avenues for therapy.
In this project we focused on the role of ventral Tegmental area (VTA) GABA neurons in alcohol self administration. Dr. Steffensen’s previous research found VTA GABA neurons accelerate their firing rates when associated with rewarding or reinforcing behavior such as intracranial self-stimulation (ICSS) (Steffensen, Lee et al. 2001). ICSS involves stimulating areas of the brain associated with pleasure such as the nucleus accumbens invoking a sensation of pleasure. In Steffensen, Svingos, et al (1998), they recently characterized a homogeneous population of VTA non-DA neurons that are distinguishable from DA neurons electrophysiologically, anatomically, and pharmacologically from DA neurons. These non-DA neurons were determined to be GABA-containing projection neurons with widespread distribution to limbic structures. VTA GABA neurons modulate the mesocorticolimbic dopamine (DA) system which is implicated in both natural and drug rewards. The firing rate of VTA GABA neurons is markedly sensitive to acute intoxicating doses of ethanol (i.e. passive administration) and undergoes adaptation to chronic ethanol (Gallegos, Criado et al. 1999). Most importantly, VTA GABA neurons accelerate in anticipation of rewarding stimuli, such as ICSS (Steffensen, Lee et al. 2001). We have recently found that VTA GABA neurons accelerate in association with heroin and sucrose self-administration. Taken together, these findings indicate that VTA GABA neurons play a role, whether contributory or reflective, in rewarding behaviors. Our proposed model would evaluate the effect of low-dose ethanol on the VTA GABA neuron’s discharge rates in freely-moving rats.
We implanted rats with electrodes in the VTA for chronic recording of VTA GABA neurons and a jugular catheter for administration of intravenous ethanol at low doses (0.10 mg/kg). We hoped to set up an operant-type chamber in which the rat would nose-poke to receive a desired stimuli, such as a low-dose of ethanol. Complications from the implant procedure made this impossible. After several attempts, we decided we didn’t have the means to safely implant the catheter into the rats for the purpose of this study.
I redirected my efforts into a new study titled, “Pilot Study: Ameliorization of seizures by Botulinum Toxin.”
The current treatment of epilepsy, whether congenital or acquired, has been treated with phenobarbital, carbamazapines and lamotrigine. While these drugs are therapeutically useful, the line between the suggested dose and the lethal dose is very thin. Alternative treatments for epilepsy are eagerly being sought.
Botulinum B, a member of the family of Botulinum toxins, has recently come to be considered as a candidate for treating epilepsy. Botulinum toxins (clinically known as Botox) are known to interfere with vesicular fusion with the presynaptic membranes in neuromuscular junctions. It is use clinically to treat for muscle spasms (Chapman et al., 2007), hyperactive sweat glands (Glaser et al., 2007), and wrinkles (Kim et al., 2007).
Research has found that Botulinum B increases the threshold for seizures in rats when administered directly into the hippocampus (Costatin et al., 2005). Botulinum toxins cleave the SNAP protein involved in fusing vesicles containing neurotransmitters to the cell membrane. Botulinum toxin is commonly supposed to be unable to breech the blood-brain-barrier (Simpson, 2004). However, anecdotal observations by Dr. Gary Borodic have noted that patients who recently underwent Botox procedures in the periorbital region have altered moods. This suggests that the toxin may be transported into the cranium.
To test if the clinical form of Botulinum can indeed cross the blood brain barrier, we replicated the kindling model of epilepsy (Costatin et al., 2005) and administered known therapeutics (Pentobarbital) for epilepsy to evaluate our model for validity. Then, we administered the Botulinum toxin periorbitally (on the forehead).
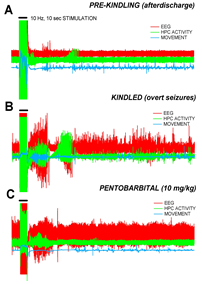
The kindling model involves implanting a stimulating electrode into the dentate gyrus of the hippocampus, a known locus for epilepsy. We then stimulated the rat at regular intervals and at slightly increased voltage until the rat exhibited seizure like behaviors and electroencephalography (EEG) recordings.
The figure to the right shows the EEG recordings of an animal before the kindling has taken affect, a characteristic seizure after kindling and the effect of Pentobarbital on these seizures.
The animal subject responded well to the implants and to the kindling procedure. Several study groups and control groups were then kindled and had the toxin administered periorbitally. Unfortunately, no significant decrease was found to result from the administration of the Botulinum toxin. After these groups finished their protocol we ended the study.
I presented a poster at the 2008 Mary Lou Fulton Student Research Conference here at BYU. These studies may not have discovered a significant finding and shared it with the community but I learned a great deal about the scientific process. Perhaps most importantly, I learned that a failure doesn’t mean we haven’t learned anything. It just means we have to change the course of our study.
I am immensely grateful for the opportunity that the ORCA grant afforded me. I’m now in medical school, largely in part to these research experiences. Thank you for your support and patronage.
