Bradley Nilsson
Current literature contains many examples of the use of light to induce the synthesis of novel compounds and natural compounds. One reaction that has been extensively studied is the meta photocycloaddition of arenes to alkenes. Commonly this is experimentally treated as an inten-nolecular reaction. Problems with this approach to the photocycloaddition include low yields and extensive side reactions with competing regioselectivities. We felt that bringing the alkene and the arene together via a molecular tether prior to irradiation with UV light would increase yields and limit side reactions. Our goal is to find a way to make this reaction more synthetically useful.
We decided to use silicon as a tether. The literature has reported the use of silicon and previous work in our research group has demonstrated its usefulness in intermolecular [2 + 2] photoadditions. It is attractive as a tether because silanes can be easily prepared, and the silicon can be hydrolyzed in a separate reaction step following photolysis.
Methods
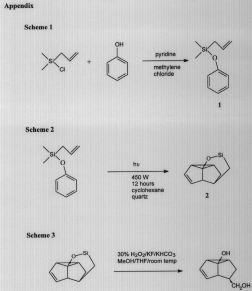
We prepared our starting material, allylphenoxydimethylsilane, according to the reaction shown in Scheme 1. (See Appendix). One equivalent each of allylchlorodimethylsilane, phenol, and pyridine are stirred in methylene chloride at about a 0.5 M concentration. Allylphenoxydimethylsilane (1) is produced in high yield and can be easily purified by distillation under vacuum using a Kugelrohr (100(C at about I Torr).
The starting material, 1, is then irradiated according to Scheme 2. The photolysis was carried out using a 45O W high pressure mercury lamp and a quartz reaction jacket. Cyclohexane is the solvent. We experimented with concentrations ranging from 0.01 M to 0.06 M. The desired product is product 2. The desired product required extensive purification. This was done by distillation, prep TLC, column chromatography, and high pressure liquid chromatography.
Product 2 is then hydrolyzed using hydrogen peroxide according to Scheme 3. This effectively desilylates product 3 and yields the diol. One equivalent of sodium bicarbonate, six equivalents each of 30% hydrogen peroxide and potassium fluoride are stirred with product 3 for 10 hours. The solvent is a 1:1 mixture of methanol and THF at a concentration of approximately 0.4 M.
Reasonable yields of the diol have been obtained. Product has been identified by 2D NMR and C 1 3 NMR analysis. We have found that by limiting the concentration of 1 during the photolysis reaction we can increase yields. At higher concentrations we tend to get more polymerization side reactions and degradation of product. We have also found that very little product is obtained if we let the photolysis run much longer than 48 hours. Large amounts of solid polymer product are obtained. Product has been obtained in as little as 12 hours of reaction time, but it seems the best yields result from between 24 and 48 hours. Up to 50% conversion has been achieved in one instance.
This project is now in its final stages. We are attempting to further characterize the photoproduct. We still need to get mass spec data and we want to derivatize the diol so we can get it to crystallize out as a solid. This will enable us to get x-ray crystallography data and thus verify the crystal structure of the product. When these steps are complete the project will be ready to submit for publication.
References
- a) Weedon, A.J. Org. Chem. 1994, 59, 1333; b) Sigamura, T. Tetrahedron Asymm. 1994, 5, 879.
- Cornelisse, J. Chem. Rev. 1993, 93, 615.
- Crimmins, Tetrahedron Lett. 1994, 1657.