Christopher M. Lee and Dr. G. D. Watt, Chemistry and Biochemistry
Ammonia is extremely valuable in the industrial production of fertilizer, plastics, explosives, dyes, and other compounds. The most plentiful and common source of ammonia comes from the conversion of atmospheric nitrogen gas to ammonia. In industry the 80-year-old Haber method is used, which is a costly process that relies on very high temperatures and pressures to catalyze the reaction.
In comparison to industrial processes, certain plants, peas and other legumes, can naturally convert nitrogen gas to ammonia at room temperature and pressure. Such plants are unique in that bacteria living in nodules on their roots symbiotically work with the plant to break down nitrogen that is absorbed into the soil in order to produce ammonia. The process of breaking down nitrogen is termed “nitrogen fixation.” Enzymes such as the nitrogenase enzyme are located within nitrogen fixing bacteria and perform this process.
The nitrogenase enzyme is composed of two identical proteins. Each protein contains an iron cluster and a molybdenum-iron cofactor. The molybdenum-iron cofactor is the site of nitrogen fixation in the enzyme. This reactive site can only fix nitrogen when homocitric acid, an organic acid molecule, is covalently bound to the molybdenum atom in the cofactor. 1,2
In this study, I isolated, purified, and identified the molybdenum-iron cofactor from Azotobacter vinelandii, a nitrogen fixing bacterial strain grown at Brigham Young University. I isolated the molybdenum-iron cofactor by following the techniques proposed by Ma et al., an acid denaturization technique used to free the cofactor. These procedures were performed under anaerobic conditions. First, aqueous solutions of molybdenum-iron protein were brought to a pH of 5.5 for five minutes. This low pH allowed the protein to unwind and release the cofactor. The cofactor was than separated from the protein through extraction into degassed 2-butanone. I next evaporated off the 2-butanone and dissolved the cofactor into n-methylformamide. By passing the n-methylformamide solution through a column packed with Sephadex G-25 powder, the molybdenum-iron cofactor was purified.’
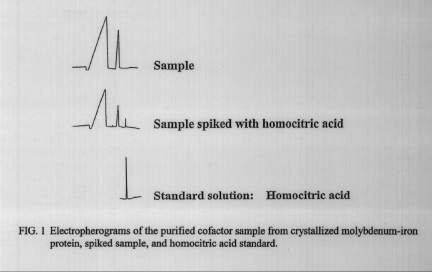
I used capillary electrophoresis to determine the absence or presence of homocitric acid in the purified cofactor, and to measure its concentration. The analysis required a 0.005 M potassium biphthalate buffer (pH 7.4), 60 cm x 5 um i.d. untreated fused silica column, and injection voltage of 28 kV. I used a UV-absorption detector set at 254 run. In order to quantitatively determine the homocitric acid concentration, I made a calibration curve based on peak areas under curves created by standard homocitric acid solutions. The standard curve allowed me to determine the unknown homocitric acid concentration in the purified extracts. I also spiked portions of the purified extracts with a known amount of homocitric acid to compare peak sizes and retention times.
Figure 1 clearly shows the absence of homocitric acid in the purified extract. After the addition of the homocitric acid spike, the retention time of homocitric acid in relation to the other peaks is also evident. The homocitric-acid peak is clearly not masked by the other peaks present. These results show that homocitric-acid dissociates from the molybdenum-iron cofactor when the cofactor is released from the protein core.
References
- Moffat, A.S. 1987. Science 257: 1624.
- Dean, D.R., et al. 1993. Journal of Bacteriology 175: 6737.
- Ma, L., et al. 1994. Journal of Biological Chemistry 269: 18007.