Jason Nielson and Dr. Merritt Andrus, Chemistry and Biochemistry
During the past year, I have actively worked at developing analogues of the anti-cancer agent kurasoin B. I have included the synthetic path that we have followed during the past year as a reminder of the proposed plan I submitted. Included in the general synthetic pathway are the percent yields that I was able to obtain for each reaction.
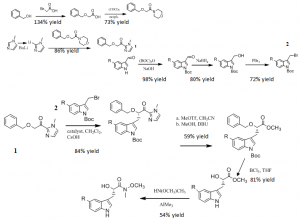
I was fairly successful at following this synthesis throughout the year. When we reacted intermediate 1 and 2 we were able to get one of the enantiomers in approximately an 85% excess. Only the enantiomer we synthesized is expected to have anticancer potential, thus it was very exciting to achieve such a high percentage of selectivity. These last few steps do not have percent yields included with them because these last steps have been completed for only a portion of the analogues.
Some problems did arise throughout the year that I had to resolve in order to continue progressing on the project. I had to experiment with the concentrations of solvent and substrate in order to get acceptable yields. Furthermore, the characterization of some of the intermediates in the project was difficult as some of the substituents of the indole substrate backbone are quite labile and thus were difficult to observe in the mass spectrometer. However, I was able to rely primarily on the NMR data I obtained in order to characterize the compounds.
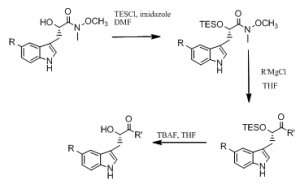
During the past year, I have also worked on developing a method to generate a wide array of indole substrates (compound 2 in the synthetic pathway). I attempted several series of experiments with the aim of generating the indole substrate I needed to create additional analogues of kurasoin B. Below is the path I attempted to follow.

First, I attempted reactions with the iodoaniline and alkyne substrate. These reactions were all successful in creating the desired indole. However, iodoanilines can be rather expensive, so I attempted to create the same indole with aminophenol. Aminophenols are quite cheap and would provide a great alternative to the iodoanilines. I thought the aminophenols would work well to create the indole substrate if I was able to make the hydroxyl group a better leaving group with tosyl chloride or triflic anhydride. I was successful in converting the aminophenol substrate to a tosylated aminophenol and to the triflated aminophenol. However, neither the tosylated aminophenol nor the triflated aminophenol reacted very well with my alkyne substrate to produce the desired indole. I attempted many different conditions, but ended up setting this part of the project to the side due to a lack of productive results.
During this past year, I successfully incorporated some unique elements of phase-transfer catalysis to develop analogues of kurasoin B. The phase-transfer catalysis methodology that I employed allowed me to generate the analogues in an 85% enantiomeric excess, indicating that over 85% of the material produced could serve as a viable anti-cancer agent. Because of the high level of enantiomeric selectivity in this synthesis, the pathway I have used to produce analogues of kurasoin B is a plausible pathway for producing these compounds if they perform well in the in vitro and in vivo assays that will be conducted in the very near future.
