Glade E. Roper and Dr. Paul B. Savage, Chemistry and Biochemistry
Septic shock is a disease that kills over 100,000 hospitalized patients in the United States every year. The mortality rate for those who fall into septic shock is between fifty and eighty percent, and despite advances in medicine this rate has remained constant over the years.1 Septic shock is caused by an inflammatory response to Gram–negative endotoxin, also known as lipopolysaccharide (LPS).
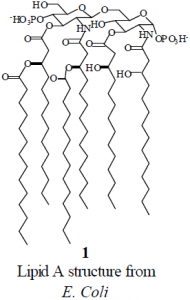
Bacteria can be covered with one of two types of membrane: Gram–positive or Gram–negative. Gram–positive bacteria are covered with a thick cell wall, which provides rigidity and which contributes to the virulence of bacteria. Gram–negative bacteria are encased in a thin cell wall that is covered by a complex outer membrane.2 Gram–negative endotoxin is a primary constituent of the outer membrane of Gram–negative bacteria, though portions of its structure vary from species to species. However, a part of LPS known as lipid A (LA), 1, is highly conserved among Gram-negative bacteria, and LA is the endotoxic component of LPS.
When LA is embedded in a membrane, the head group of LA is exposed and the long hydrophobic chains are sequestered within the membrane. However, antibiotics can still bind LA, indicating that antibiotics bind to the head group of LA. Polymyxin B, a polypeptide antibiotic, has been shown to be an effective binding agent for LA.3 Binding LA effectively detoxifies it, thus attenuating the inflammatory response that causes septic shock.4 Compounds designed to mimic the LA-binding properties of polymyxin B have been synthesized by the group led by Dr. Paul B. Savage at Brigham Young University. Experimental evidence suggests that these compounds bind LA.5
We wanted to determine what factors lead to the binding of LA by polymyxin B and related antibiotics. Specifically, we wanted to know if the binding of LA by antibiotics is driven by hydrophobic or hydrophilic interactions. Knowing what factors influence the binding of LA by antibiotics will help us to design compounds that effectively bind LA. To determine what factors are involved, we wanted to use microtitration calorimetry to determine the heat released or absorbed by the binding of LA by the antibiotics. However, LA is only soluble in water at nanomolar concentrations, and it is difficult to perform experiments on such a small scale of concentrations.
To circumvent this problem, we designed analogues of LA that have the same head group as LA, but that have unbranched hydrophobic chains. The analogues have chains of two, six, ten, and fourteen carbons. For example, the C10 analogue, 2, has hydrophobic chains of ten carbons that are attached to the head group. These analogues are much more soluble in water than LA itself, and this solubility facilitates mictrotitration calorimetry experiments.
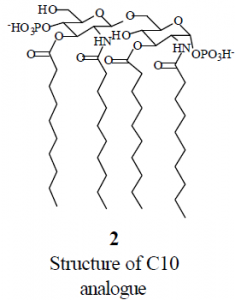
My initial proposal was that I would synthesize LA analogues with chains of two, six, ten, and fourteen carbons, and then perform microtitration calorimetry experiments at varying concentrations of the analogue to determine what factors influence the binding of antibiotics to the head group of LA. However, it quickly became apparent that the task I proposed was too great for one person to do all the work in a reasonable amount of time. The synthesis of each analogue requires an eleven– step synthesis to form an acceptor, a twelve–step synthesis to form a donor, and then six steps to join the donor to the acceptor and deprotect the final product. After some discussion with the Savage research group, I was assigned the task of synthesizing the C10 and C14 acceptors.
This project has lasted from January, 2000 to August, 2000 and may continue for another two or three months. Since the beginning of the project, I have synthesized six grams of the C10 acceptor and 1.8 grams of the C14 acceptor. I also currently have approximately five grams of C10 acceptor that is almost finished; one more step and the product will be ready to join to the donor. I also have approximately 7 grams of C14 acceptor that is five steps from completion.
While working on this project, I have refined the procedures used to synthesize the acceptors and have taught those procedures to my colleagues who are working on the C6 and C2 acceptors. I have characterized the products I have obtained, using NMR spectroscopy and mass spectrometry. One of my colleagues is currently joining the acceptors I have synthesized to the donor molecules synthesized by others. The microtitration calorimetry experiments will start in September, and though I will not be directly involved in those experiments I will continue to monitor the results.
References
- Bone, R.C.; Fisher, C.J.; Clemmer, T.P.; Slotman, G.J.; Metz, C.A.; Balk, R.A. N. Eng. J. Med. 1987, 317, 653.
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry; Wiley & Sons: New York, 1999; p 210.
- David, S.A.; Balasubramanian, K.A.; Mathan, V.I.; Balaram, P. Biochem. Biophys. Acta. 1992,1165, 147.
- Durando, M. M.; Mackay, R. J.; Linda, S.; Skelley, L.A. Am. J. Vet. Res. 1994, 55, 921.
- Li, C.; Budge, L.P.; Driscoll, C.D.; Willardson, B.M.; Allman, G.W.; Savage, P.B. J. Am. Chem. Soc. 1999, 121, 931.
