Mark Benson and Dr. Paul B. Savage, Chemistry and Biochemistry
The aim of this project is to synthesize a non-peptide lipid A-binding agent which is less toxic and simpler than polymyxin B. Such a binding agent could be used to fight sepsis, a toxic condition where the body overreacts to the lipid A portion of bacterial endotoxin released by Gram-negative bacteria.
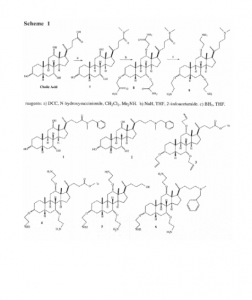
The original proposed synthesis involves three steps (Scheme 1). However, some of these steps did not work, and we had to take alternate routes.
The first step was to attach a UV-active group (benzymethylamine) at the carboxylic acid site of cholic acid. Having a UV-active group enabled us to help identify our target molecule via UV absorption during reactions. We successfully produced the amide derivative (1) in good yield.
The second step involved adding three groups at the hydroxyl sites on the amide (1). Attaching these groups, called linkers, was the hardest part of the project. We attempted to add 2-iodoacetamide at the three hydroxyl sites using the Williamson ether reaction; however, none of these attempts was successful. We tried various conditions but still could not add the linker onto all three hydroxyl sites.
We decided to switch from forming ether linkers to forming ester linkers. Formation of the ester linker requires less severe reaction conditions as compared to the synthesis of ethers, and gave a better chance of forming. We reduced the amide (1) to an amine (2) at this point in the synthesis, which normally is the last step, because reducing would cleave the ester linkers. The two groups we attempted to add were BOC-glycine and BOCalanine. We were successful in attaching three BOC-glycine groups, yet the molecule was not stable and was subject to decomposition.
We next tried using allyl groups to form linkers. A benefit of using allyl groups is that two different linkers could be formed by using different pathways starting from the same allyl group. The allyl group could be altered to form either two or three carbon chain linkers by ozonolysis or hydroboration-oxidation respectively. We were able to add two allyl groups, but not the third. However, a co-worker in the lab, Eric Meredith was able to attach three allyl groups to a trityl ether derivative (3). The reason why the allyl groups will add on the trityl ether derivative (3) and not the amide (1) is not known at present.
Future synthesis proves to be straightforward, continuing with the trityl ether. The linkers will be formed from the allyl groups (4). The trityl protecting group will then be removed (5) and a UV-active linker (benzylmethylamine) will be attached, yielding the final product (6).