Adam L. Lunceford and Dr. Steven W. Graves, Chemistry and Biochemistry
The Na,K-ATPase is a membrane-bound enzyme found in all cells. This enzyme is composed of an a subunit, which is responsible for the enzyme’s catalytic activity, and a b subunit, which assists in the binding of ions. The three main isoforms of the a subunit are designated a1, a2, and a3. The Na,K-ATPase is responsible for maintaining the proper balance of sodium and potassium ions in the intracellular and extracellular fluids Inhibition or decreased activity of the Na,K-ATPase enzyme has been implicated in the development of cataracts. Without the proper ionic balance, lens cells may be damaged and experience protein leakage, which then leads to the formation of opaque plaques, or cataracts. Our objective was to first determine the distribution of the isoforms in the ocular lens and then to investigate their possible hormonal regulation in vitro.
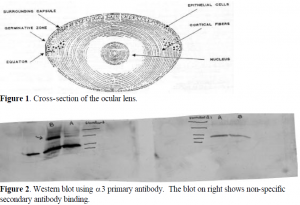
We analyzed the distribution of the three a subunit isoforms in the three layers of tissue in the human and bovine lens. These layers are the epithelial layer, the cortex, and the nucleus. Figure 1 illustrates theses layers and shows how epithelial cells differentiate to form cortical cells. The lenses were first dissected into these layers, and the tissue was homogenized to free the Na,KATPase from the cells. To determine the distribution of the Na,K-ATPase in the lens, we used a technique called SDS-PAGE. In this technique, a protein solution is exposed to sodium dodecyl sulfate (SDS), a compound that has a hydrocarbon tail that interacts with the proteins and a sulfate head that coats the outside of the proteins with a negative charge. The protein solution is then loaded onto a polyacrylimide gel (PAGE) and an electric potential is generated across the gel. The smaller proteins migrate to the positive end of the gel more quickly than the larger proteins, and this allows the proteins to be separated according to size. On each gel that we ran, we had protein solutions from the epithelial layer, cortex, and nucleus in parallel lanes.
Once the lens proteins were separated in the gel, they were transferred to a nitrocellulose membrane using an electric potential to push them onto the membrane. At this point, we used a technique known as Western blotting. The membrane is first exposed to a milk solution for one hour. The proteins in the milk bind to all the unoccupied spots on the membrane and prevent non-specific binding of the Na,K-ATPase antibodies. Using primary antibodies specific for each of the three a subunit isoforms, we incubated the membranes for one hour, and after rinsing them, added the secondary antibody. This antibody recognizes and binds to the primary antibody. In addition, it contains an enzyme, horseradish peroxidase, which reduces a chemical solution causing it to fluoresce. The light emitted then exposes photographic film producing bands in the lanes where Na,K-ATPase is present.
Using these techniques, we found that a1 and a3 were present in the lens in fairly large concentrations. They are found mostly in the epithelial layer with small amounts appearing in the cortex virtually none showing up in the nucleus. One complication we encountered was multiple bands resulting from non-specific binding of secondary antibody. To confirm that nonspecific binding was the cause of these bands, we ran two parallel gels, and with one membrane we used primary (a3) and secondary antibodies, while with the other we used only secondary antibody. Figure 2 shows the resulting Western blot. Fortunately, the non-specific bands appear much lower on the gel than does the Na,K-ATPase, so they do not interfere with each other.
Once the distribution of isoforms was determined, we began the long process of developing a protocol to perform primary cell culture using the epithelial layer of bovine lenses. This required a lot of trial and error and therefore took a long time. We found that the best way to transport eyes was in a buffered saline solution on ice. Mild bleach solutions reduced the chances of bacterial contamination in cell culture, but they also damaged the delicate lens cells and reduced the efficiency of primary culture. The media in which the cells grow best consisted of: Minimum Essential Media Eagle, 10% fetal bovine serum, and 1% streptomyocin and penicillin. Cells grew well for about six weeks and had to be passaged at least once each week on average. At the end of this period, however, the cells got old and no longer grew well in culture. Phosphate buffered solution (PBS) with 2 mM EDTA had to be used to passage cells instead of trypsin to avoid damaging the membrane-bound Na,K-ATPase. We are currently exposing the cells to progesterone to see if it has a significant effect on the Na,K-ATPase, but we do not have conclusive results as yet. Much time and effort has been spent on finding the best conditions to get the cells to grow. Much work has yet to be done.