Mari Evans and Dr. Milton L. Lee, Chemistry and Biochemistry
The isoenzyme thymidine kinase (TK1), found in human serum, has been shown to be a cancer tumor marker. It has demonstrated both diagnostic and prognostic value in the treatment of breast cancer.1 Currently TK1 levels are measured by radioimmunoassay and enzyme-linked immunosorbant assay (ELISA). Radioimmunoassay is not specific for TK1, is relatively expensive, uses radioactive materials and is labor intensive. Immunoassays of TK1 have been developed which are selective and sensitive but they are still labor intensive. A new assay is needed to make the information TK1 can provide accessible to physicians who treat and diagnose cancer. Capillary electrophoresis (CE) would be well suited to medical application because of the high degree of automation, small sample requirements and long lifetime of columns. Because of the narrow volume of the capillaries that are used, high speed, high resolution seperations can be achieved from minimal detection volumes. My research was a small step aimed at developing a test for TK1 that could be developed commercially.
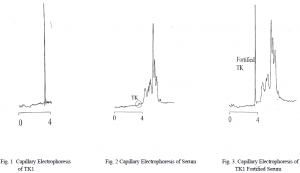
This research identified conditions for the detection of TK1 by capillary electrophoresis coupled with UV-Vis detection. Because the concentration of TK1 in serum is very low the conditions for identifying TK1 were first found using purified TK1. TK1 was purified according to the methods of O’Neill et all.1 The results were obtained using a fused silica capillary, 60 cm x 50um i.d., and phosphate buffer. The peak became sharpest at high pH, pH 10.2. The sample was injected at a voltage of 10 kV for 0.1 min. and the run voltage was 28 kV. The detection wavelength was 200nm (Fig. 1).
In hopes developing a method to quantatize the TK1 in serum or at least be able to estimate the relative level of TK1 in a serum sample, several serum samples with varying TK1 levels were run under the above conditions. However, due to the very small amount of TK1 in serum compared to the many proteins in the blood, even the sample with an extremely elevated level of TK1 only produced a small rough peak with an identical retention time to the peak seen in the purified TK1 run (Fig. 2). The difference between normal serum and serum with elevated TK1 levels was not clearly distinguishable. The small rough peak was suspected to be TK1. In order to confirm this suspicion the serum was fortified with purified TK1 (Fig. 3).
Clearly a more sensitive detection is needed in order to develop an assay for TK1 that can be commercialized and made available to clinicians. Perhaps laser induced fluorescence (LIF) detection would add the sensitivity needed to develop a successful assay for TK1 using capillary electrophoresis.
References
- O’Neill KL, Abram WP, McKenna PG. Serum Thymidine Kinase levels in cancer patients. Ir. J. Med Science 1986; 155272-4
- Acknowledgement and thanks to Roopa Singh and Dr. Kim O’Neill
