Josh Nelson, Seth Stapley and Scott Steffensen, Psychology
Introduction-
Drug addiction has long been a major detriment to society. Its pervasive effects can tear apart the lives of addicted individuals. Many of these individuals go without seeking professional help. In 2015, 22.7 million Americans (8.6 percent) needed treatment for a problem related to drugs or alcohol, but only about 2.5 million people (0.9 percent) received treatment at a specialty facility. This may be due to inadequate treatment methods and therapies in use by professionals. The goal of this project is to better understand the mechanism of dopamine (DA) transmission in the brain in order to develop more effective treatment methods for addiction.
Initially, this project intended to investigate the effects Mefloquine (MFQ) on DA release. However, some initial experiments steered research towards another avenue. Specifically, the decision was made to investigate the role of intracellular calcium signalling on Methamphetamine (METH) effects on DA release. The mesolimbic system is an epicenter for DA release and METH has multiple effects on DA release within this region, such as increasing vesicle release, increasing DA accumulation in presynaptic neurons, and decreasing DA reuptake (Chu et al, 2008; Fleckenstein et al, 2009). Our lab has previously shown that METH may induce increased DA release through activation of a sigma receptor, IP3R mechanism involving the release of intracellular calcium stores. The study we performed verifies that the effects of METH involve the activity of this proposed mechanism, thereby showing that METH increases vesicular and non-vesicular DA release through multiple mechanisms.
Methodology-
Coronal brain slices were obtained from male C57 mice who were bred and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were anesthetized with 5% isoflurane, decapitated, and brains were rapidly extracted and sectioned into 300μm slices in ice-cold artificial cerebrospinal fluid (ACSF). Slices were placed in a chamber containing room temperature ACSF bubbled with carbogen, then transferred to a recording chamber with continuous ACSF flow (2.0 mL/min) at 34-36 °C. Carbon fiber electrodes (CFEs) were placed into the NAc core and dopamine release was evoked every 2 min by biphasic electrical stimulation (4 ms pulses, 10 pulse, 350 μA, 20 Hz) from an ACSF-filled micropipette placed 100-200 μm from the CFE. Cyclic voltammograms were recorded every 100 msec (10 Hz) with ChemClamp potentiometers and recordings were performed and analyzed using LabVIEW-based Demon Voltammetry software. Stimulated DA release was detected to verify CFE placement, and after electrically-evoked DA release did not vary by more than 5% for five successive collections, the electrical stimulation was turned off and voltammograms were recorded at 0.5 Hz for 3 hrs. All recordings were performed with the pharmacological agent added at 30 min and methamphetamine at 60 min from the start of recording. Each pharmacological agent was dissolved in stock solutions and then diluted into ACSF at specified concentrations.
Results-
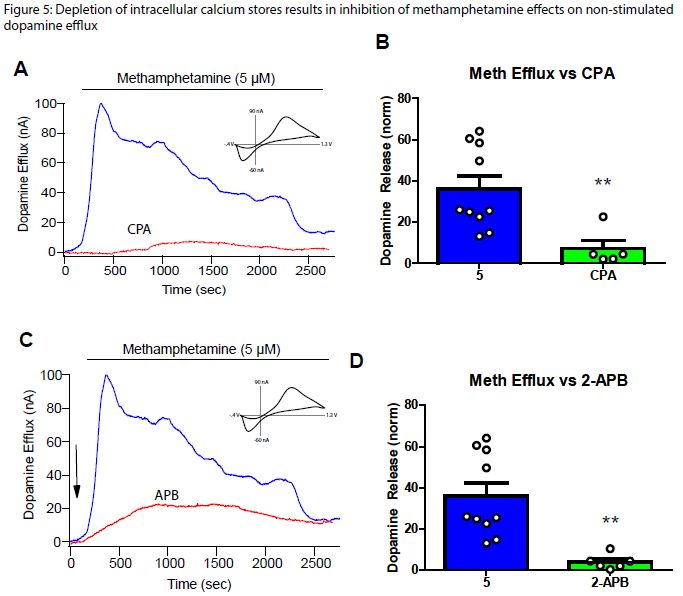
Since little is known about the mechanism of intracellular calcium on DA release alone and METH effects on DA release, bath application of cyclopiazonic acid (CPA) was used to induce depletion of intracellular calcium stores. The IP3 receptor inhibitor 2-aminoethoxydiphenylborane (2-APB) was also used via bath application to observe the effect on non-vesicular DA release. CPA and 2-APB reduced DA efflux significantly when compared with the METH controls (Figure 1). This shows that intracellular calcium stores are needed for effective non-vesicular DA release (Figure 1A, 1B). Since 2-APB reduces efflux (Figure 1C, 1D), intracellular calcium release likely occurs via activation of the IP3R via METH activation of the sigma receptor.
Discussion-
Previously, we have shown that CPA stimulated release with METH and 2-APB stimulated release with METH reduces vesicular DA release while stimulated release with CPA or 2-APB alone does not decrease DA release (Data not shown). Combining this data with our study on non-vesicular DA release shows that METH effects on DA release involve enhancement of the IP3R pathway. Previous studies show that blocking the sigma 1 receptor decreases METH effects on DA release (Hedges et al, 2018). METH is a sigma receptor agonist (Maurice and Su, 2009) and sigma receptors are involved in the release of intracellular calcium by activating IP3R’s (Ruscher and Wieloch, 2015). This implies that the increase of intracellular Figure 1 calcium via activation of the sigma receptor is one mechanism by which METH increases DA release through both vesicular and non-vesicular means.
Figure 1
Conclusion-
This study has revealed an additional mechanism by which METH affects DA release. Meth activates sigma receptors which initiate a cascade to induce the release of intracellular calcium stores through IP3R activation. This increased intracellular calcium increase the release of DA, in addition to other mechanisms.
References-
Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, et al (2008). Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol 590(1-3): 105-110.
Fleckenstein AE, Volz TJ, Hanson GR (2009). Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56 Suppl 1: 133-138.
Hedges DM, Obray JD, Yorgason JT, Jang EY, Weerasekara VK, Uys JD, et al (2018). Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway. Neuropsychopharmacology 43(6): 1405-1414.
Maurice T, Su TP (2009). The pharmacology of sigma-1 receptors. Pharmacol Ther 124(2): 195-206.
Ruscher K, Wieloch T (2015). The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration. J Pharmacol Sci 127(1): 30-35.