Elizabeth Mahoney, Alonzo Cook, Chemical Engineering Department
Introduction
Neuropathy, disease, and trauma to the peripheral nervous system cause devastating effects. These effects can include both a loss of sensation and a loss of motor control. Due to the newness of the field of regenerative medicine, scientists are only beginning to understand how nerves regenerate. It is known that when damage occurs to the nerves, the body triggers a regeneration process. Unfortunately, this process is slow and the level of sensation that returns and motor control varies from person to person. Some options involving autografts and allografts have been employed to help the nerve endings regenerate to all distal ends. When these options cannot be used, new nerve fibers grow disproportionately, and tissue is left without innervation. In the case of trauma, scar tissue simply fills in the space the best it can. If space is preserved, site appropriate tissue more readily regrows and fills in the gaps. We seek to improve the speed and quality of recovery of damaged peripheral nerves through the use of a multichannel porcine-derived urinary bladder matrix conduit (UBM), produced by ACELL, Inc.
Methodology
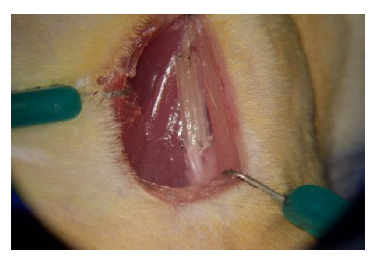
Using sterile techniques, we performed surgeries on rats under anesthesia using a protocol approved by the Institutional Animal Care and Use Committee at BYU. We first measured the electrical current of the healthy nerve, then transected the nerve. Immediately following the transection, we sutured either a conduit (Figure 1) or the transected nerve in reverse direction to both ends of the nerve. We had four groups: an autograft group (where the transected nerve is flipped 180 degrees and sutured to both ends of the nerve), a single-channel UBM conduit group, a multi-channel UBM conduit group, and a silicon conduit group. The autograft is the control for comparison, as reversing the direction of the autograft provides a comparable situation to harvesting a graft from elsewhere in the body, as is commonly done in clinical practice. We then performed weekly foot fault testing each week for six weeks to measure the functionality of the hind leg over time. Foot fault testing was done by allowing the animals to roam freely on a wire mesh grid while being filmed for five minutes. Later, the videos were analyzed to count the number of foot faults that occurred during 50 steps taken by each leg. A full fault was recorded if the animal’s hind limb fell through the opening in the grid and touched the floor, whereas a partial fault was recorded if the foot did not touch the floor. The electrical conductivity of the muscles of the hind leg were also measured at the start point and end point at six weeks. At the end point the animals were euthanized and the nerves were harvested for cell staining. The nerves were fixed in Karnovski’s fixative for 24 hours, and then stored in PBS before being fixed in resin. A microblade was then used to cut slices for microscopy. The stained cells showed the types of cells that grew inside of the conduit.
Results
We have completed three of the four groups, and plan to do the last group, the silicone conduit group, starting in January. Our study was complicated by the moving of our animals into another building while our original building was renovated. Later in the study, when the renovations were complete, we were required to move back in order to have access to important equipment during our animal surgeries. These events postponed certain completion dates for our study, so we were not able to complete the study by the end of this year like we had anticipated. We have, however, seen positive results in our behavioral testing data hinting that the rats in the UBM conduit groups had a significant recovery over the six-week period.
Discussion
Our current plan is to perform surgeries on the rats in the silicone conduit group in January, so that we can complete the conduit group by the end of February, 2019. We have already completed a quarter of the gait analysis video coding and have sent the autograft and multi-channel conduit nerves for the cells to be analyzed by histology. We hope to have all of the data analyzed by April and submit the study for publication in the summer.
Conclusion
Our group has studied peripheral nerve regeneration for three years. We have investigated the effects of lysophosphatidylcholine, nerve growth factor, silicone conduits, and now we are investigating the effects of a urinary bladder matrix (UBM) conduit. The goal of this research is to find an alternative to the autograft, which is the current clinical standard. One study has shown that single-channel UBM conduits have potential to produce similar results to an autograft, and we are investigating to see whether multi-channel conduits made of UBM can also produce similar results.
Figure 1 – Hind leg of rat with a single channel conduit implanted.