Lily H. Carlson, Prof. Daniel H. Ess, Chemistry Department
Our group is interested in identifying the unknown mechanisms of main-group C-H functionalization reactions. In the long term, our goal is to use computational chemistry tools to develop general principles on mechanisms, intermediates, reactivity, and selectivity for hydrocarbon C-H functionalization reactions by p-block main-group compounds as well as provide prediction of new catalysts and reactions. However, in providing these predictions, it remains unclear if different mechanistic pathways and intermediates will be predicted if examined in a complete solvent sphere (see Figure 1 for technical details). This is especially important for non-aqueous solvents such as carboxylic acids and sulfuric acid. To examine alkane C-H functionalization mechanisms in complete explicit solvent we continued the development of a suite of utilities to carry out DFT/molecular mechanics (MM) molecular dynamics calculations.
Our research group has already worked to develop a C++ program called DynSuite to simulate molecular dynamics. While DynSuite has the capacity to simulate molecular dynamics, it was not capable of modeling molecular dynamics within solvent and current solvation models lack the ability to predict and examine if different mechanistic pathways and intermediates are produced when surrounded by solvent. As such, we needed to develop our own model.
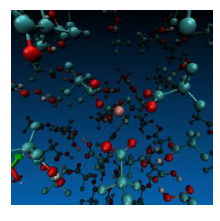
To initialize a solvent container, we found Packmol1 a Fortran program that proved to be effective at packing solvent into a container with solute. By integrating Packmol with DynSuite we were able to at least initialize a variety of solvent containers (see Figure 2). At this point we needed to add functionality to DynSuite to handle the dynamics of the solvent molecules. This included using a velocity distribution to give each solvent molecule an appropriate starting velocity. At this point we were able to produce a sphere, cube, or rectangular prism of solvent around a solute molecule and apply dynamics (see Figure 2).
However, we still needed to handle the solvent-surface interaction. Our initial attempt at solvent-surface interaction is to identify when the center of mass of a solvent molecule is projected to exit the container and reverse the velocity of the solvent to keep it within the bounds of the container. Further research will be done to evaluate this method, and to develop more accurate models that will conserve the energy of the system. The continuation of this research will shed further light on main-group metal alkane functionalization reactions in highly acidic carboxylic and sulfuric acid solvents.
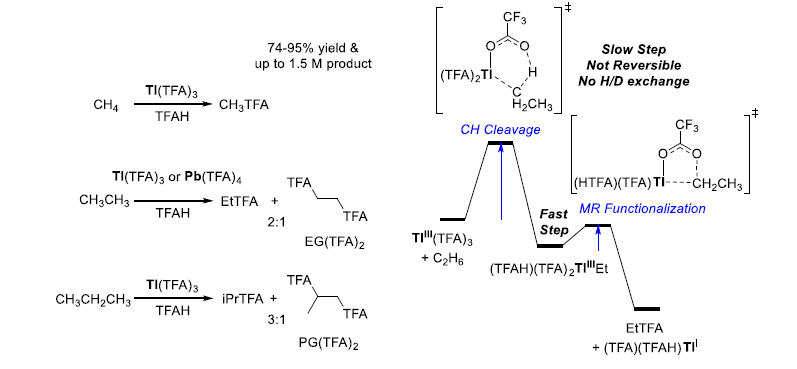
Figure 1 – Serendipitous experimental discovery demonstrated that main-group metals TlIII(TFA)3 and PbIV(TFA)4 promote C-H oxygen functionalization in trifluoroacetic acid (TFAH) of methane, ethane, and propane separately or as a mixture at ~180 ºC, in high selectivity to the corresponding trifluoroacetate esters of methanol (MeTFA), ethanol (EtTFA), ethylene glycol (EG(TFA)2), isopropanol (iPrTFA), and 1,2-propylene glycol (PG(TFA)2)2. Because TlIII can induce electron transfer with hydrocarbons, CoIII(TFA)3 oxidizes methane by a known radical mechanism, and other literature precedence for PbIV, this experimental discovery of alkane oxidation was initially assumed to result from a radical reaction mechanism. However, our density functional theory (DFT) calculations provided key evidences to support a C-H activation and metal-alkyl functionalization mechanism.3 However, it remains unclear if different mechanistic pathways and intermediates will be predicted if examined in a complete solvent sphere. This is especially important for non-aqueous solvents such as carboxylic acids and sulfuric acid.
Figure 2 – An example of TlIII(TFA)3 in a large box of TFAH solvent after a long period of DFT/MM MD equilibration produced by DynSuite.
1 http://www.ime.unicamp.br/~martinez/packmol/
2 Hashiguchi, B. G.; Konnick, M. M.; Bischof, S. M.; Gustafson, S. J.; Devarajan, D.; Gunsalus, N.; Ess, D. H.; Periana, R. A “Main-Group Compounds Selectively Oxidize Mixtures of Methane, Ethane, and Propane to Alcohol Esters” Science 2014, 343, 1232-1237.
3 Gustafson, S. J.; Fuller, J. T. III; Devarajan, D.; Snyder, J.; Periana, R. A.; Hasiguchi, B. G.; Konnick, M. M.; Ess, D. H. “Contrasting Mechanisms and Reactivity of Tl(III), Hg(II), and Co(III) for Alkane C-H Functionalization” Organometallics 2015, 34, 5485-5495.