David D. Brosnahan and Dr. Gerald D. Watt, Chemistry and Biochemistry
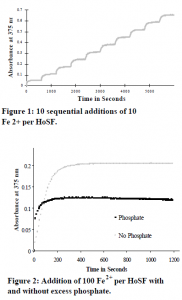
I performed a ferritin iron reconstitution experiment, making 10 sequential additions of 10 ferrous ions per ferritin molecule at 10- minute intervals (see Figure 1). Monitoring the reaction at 375 nm, I observed a fast initial absorbance change representing iron oxidation. After the initial oxidation, I observed a gradual increase in absorbance that was complete in 10 minutes. I fit the plot of the change in absorbance versus time for each addition of iron to a double exponential equation (dA/dt = -A1ekt + -A2e-kt + c). The double exponential consisted of two negative terms. k1 has a relatively large value and represents a fast initial reaction. k2 is small and represents a slower sequential reaction.
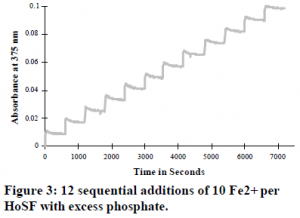
I made one addition of 100 ferrous irons per ferritin molecule in the absence of phosphate and then in the presence of 1 mM phosphate. I compared the rates of iron oxidation for the reaction with and without phosphate (see Figure 2). I observed that cellular levels of inorganic phosphate caused the initial rate of absorbance change to increase 2-fold. The presence of inorganic phosphate caused the final molar absorptivity of the reconstituted ferritin solution to be 2-fold less than a similarly reconstituted ferritin solution with no phosphate present. The gradual increase in absorbance after the initial iron oxidation with the addition of 100 ferrous irons per ferritin molecule was not observed.
I then conducted a ferritin iron reconstitution experiment in the presence of inorganic phosphate making 12 sequential additions of 10 ferrous ions per ferritin molecule in 10-minute intervals (see Figure 3). I observed a fast initial absorbance change and then a gradual decrease in absorbance, which finished after 10 minutes. The total absorbance change per addition of 10 irons per ferritin molecule in the presence of 1 mM phosphate was half that of the absorbance change per step without phosphate present. I fit the plot of the change in absorbance versus time for each addition of iron to a double exponential equation (see Figure 4). This time the double exponential consisted of a negative term and a positive term (dA/dt = -A1e-kt + A2e-kt + c) (see Figure 5). k1 is large and represents a fast initial reaction and k2 is small and represents a slower sequential reaction.
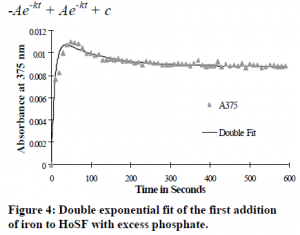
The gradual increase in absorbance in the reaction without phosphate and the gradual decrease in absorbance in the reaction with phosphate is accounted for by differences in total molar absorptivities of the ferritin iron core particles. It is apparent that phosphate decreases the molar absorptivity of the ferritin iron core particle. The intermediate iron species bound in the ferroxidase center has an equal molar absorptivity for both phosphate present and phosphate absent environment. As the iron in the ferroxidase center hydrolyzes, migrates, and nucleates in the ferritin cavity; the molar absorptivity of the resulting final product is either a little higher than the ferroxidase bound iron (phosphate absent) or the molar absorptivity is a little lower than the ferroxidase bound iron (phosphate present).
Analyzing the graph of the ferritin iron reconstitution experiment that show the absorbance change with the addition of 100 irons per ferritin molecule in the presence and absence of phosphate, the distinct second reaction is not observed. This is explained as only 6 to 8 out of the 100 iron molecules are bound in the ferroxidase sites of horse spleen ferritin at any one time. The other 92 to 94 irons mask the rearrangements of these iron molecules.
It appears from the data that the first 6 to 8 iron molecules bind quickly to the ferroxidase site and then form an active site where the oxidation of the other irons can take place. The other irons are quickly oxidized then nucleate in the ferritin iron core. These 6 to 8 iron molecules bound in the ferroxidase centers of the heavy subunit are then slowly hydrolyzed and migrate and nucleate in the ferritin core. It is possible that each iron is bound, oxidized, hydrolyzed, and released from the ferroxidase center. Conversely, iron oxidation could occur at the ferroxidase center and the surface of the iron core particle independently. Further study is necessary to elucidate the steps in ferritin iron deposition. Performing iron deposition reactions in the presence of phosphate provides a clearer view of these steps.
