Steven M. Bray and Professor Steven M. Bray, Chemistry and Biochemistry
Introduction and Project Aim
G-protein signaling is one of the major pathways which cells utilize to transmit messages. The signaling pathway consists of a receptor coupled to a G-protein, which activates secondary messengers. Signaling molecules, such as hormones and neurotransmitters, bind receptors on the cell surface, which in-turn activates G-proteins by dissociating them into a and bg subunits. These subunits bind to other molecules, secondary messengers, which further propagate the signal.
Phosducin-like protein (PhLP) is a cytosolic protein expressed throughout the body yet its function is not currently well known. PhLP is 60% homologous to phosducin (Pd), a protein known to regulate G-protein signaling in the retina. Phosducin binds bg subunits of G-proteins, thereby inhibiting their ability to activate second messengers. Because of the similarity of PhLP to Pd, PhLP is predicted to be an important G-protein regulator.
In the course of this project, I studied PhLP regulation of Angiotensin II (AngII) signaling. AngII is a hormone, which binds receptors and proceeds through a G-protein signal pathway.2 One of the many effects of AngII is growth of vascular smooth muscle cells. When the growth of these cellsis not closely regulated it can lead to high blood pressure and heart disease. In this study I used Chinese hamster ovary (CHO) cells that over expressed PhLP to study its effect. CHO cells use similar signaling mechanisms and are a well-established model of G-protein signaling.
Results
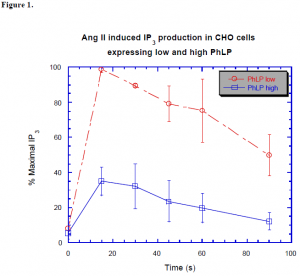
Figure 1. shows the effect PhLP has on AngII stimulation. The CHO cells were stably transfected to express either high or low amounts of PhLP. The cells were stimulated with AngII and then harvested at different time points. The high expressing cells inhibited the signal by nearly 70%. The inhibition was measured utilizing 3H labeled IP3 to monitor the signal propagation. IP3 is a second messenger whose level in the cell is quickly affected by AngII.
We performed similar experiments with phosducin to see if it behaved the same as PhLP and discovered that Pd did not inhibit IP3 levels. When Phosducin is phosphorylated it becomes inactive and cannot bind bg subunits. When phosducin’s phosphorylation site was mutated the phodsucin behaved similar to PhLP. (Data not shown) These data suggest that Pd is phosphorylated and inactive in the CHO cells. It further indicates that PhLP is not deactivated by phosphorylation. Thus, Pd and PhLP seem to have differing functions and modifications although they are very similar.
After seeing that PhLP inhibited signaling, we wanted to discover its mechanism of action. I performed an experiment to determine whether PhLP bound bg before or after AngII was introduced. Prior to AngII stimulation the G-protein has a and bg subunits bound to GDP. Upon stimulation the GDP-a separates from bg and binds GTP, which is later hydrolyzed to GDP. According to the hypothesis, if PhLP bound bg before stimulation of AngII it would disrupt the heterotrimer and GTP would not be bound. To analyze GTP binding we performed an experiment using radiolabeled GTPgS, which is a nonhydrolyzable form of GTP. The results showed that both the high and low expressers of PhLP activated the same amount of GTP. This implies that the PhLP binds bg after activation of the G-protein.
Conclusion
The data indicate that phosducin-like protein binds to bg subunits of G-proteins and inhibits signaling. The bg seems to bind only after the G-protein is activated. This conclusion is consistent with the similarities seen between phosducin and phosducin-like protein, yet our data show that the two proteins do not act in entirely the same way.
References
- Thibault, C.; Fang Wang, J.; Charnas, R.; Mirel, D.; Barhite, S.; Miles, MF. J Biol Chem. 1997 May
9;272(19):12253-6. - Touyz, RM.; Deng, LY.; He G.; Wu XH.; Schiffrin, EL.; J Hypertens. 1999 Jul;17(7):907-16.
