Nathan Keyes and Dr. John Price, Department of Chemistry and Biochemistry
Abstract
Calorie restriction is of prime importance to age related physiology and pathophysiology. It is well established that calorie restriction extends lifetime, however the full mechanism of why this happens is not completely understood. In this study, we attempted to gather kinetic proteomic data on the effects on physiology by Calorie restriction. Specifically, we focused on the proteomic changes that would occur over a period of adjustment from an ad libitum diet to a calorie restricted diet in mice. 30 mice were randomly assigned to two different diets with varying concentrations of carbohydrates, fats and proteins. After a few weeks to adjust to the diets, the mice were given only 60 percent of their normal diets, a standard life extending percent for calorie restriction. Mice were then injected with deuterium enriched saline (100 percent D2O) to bring their volume by mass 5 percent deuterium. The mice were then sacrificed in two time cohorts, week one and week two. Mice were dissected, and tissues were collected and instantly frozen on dry ice. Liver samples were later trypsin digested and ran on an LTQ Orbitrap mass spectrometer. Mass spectra were quality controlled and cataloged.
Overview
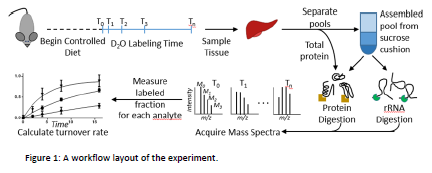
Our project was patterned after several other studies performed in the Price laboratory. In these experiments, the mice underwent an adjustment period of ten weeks of calorie restriction diets to allow them to establish homeostasis. After equilibrating, the mice were labelled with deuterium in the form of deuterated water and sacrificed at specific time points. Liver tissue from each of the mice was prepared and ionized on a Qtof Mass Spectrometer, obtaining the MSMS data. This data was analyzed through a program called “DeuteRater”, which outputs protein turnover information. After data acquisition, differences in protein turnover and quantities was compared between all of the dietary cohorts, focusing particularly on differences in the ribosomes of each cohort. In our experiment, however, we did not wait for the mice to equilibrate, but completed the time points during the adjustment phase. The purpose was to attempt to get a snapshot of the cellular starvation responses from a proteomic perspective.
Outcomes
The experimental portion of our project had a few complications throughout its duration, but overall was a success. The biggest of the complications was when one of our researchers forgot to go feed the mice their allotted meal for the day, adding an additional variable to our analysis. However, we were able to come up with several solutions that helped salvage our data. The second big complication involved deuterium enrichment rates. Several of the mice blood samples showed decreased levels of deuterium enrichment post sacrifice, making rate calculations less accurate.
As for the outcomes of our experiment, we were able to painstakingly collect several gigabytes of kinetic data on these transition state mice. This data has been cataloged and organized on the Bensen building’s hard drives. All that needs to be done is to put together a physiologically relevant story from the data to be published. We have a diagram of anticipated proteome changes that agree with previous literature.
I was able to present some of our preliminary findings at two different research conferences. The first was at US HUPO (Human Proteome Organization) in San Diego in March, and the second at BYU’s Spring Research conference.