Tania Nance and Dr. Paul Savage, Department of Chemistry and Biochemistry
Introduction
About 50 million patients worldwide are intubated annually with endotracheal tubing to open the airway, to deliver oxygen, medicine or anesthesia, to support breathing, to remove blockages from the airway and to protect the lungs from aspiration. Along with their medical uses, they also provide an abiotic surface on which bacteria and fungi form biofilms. A biofilm is any group of microorganisms in which cells stick to each other or to a surface. The structure of the biofilm protects it against against antibiotic attack. The release of endotoxins and planktonic organisms from the biofilm can travel to the lungs and cause damaging inflammation and infections. In many cases it leads to pneumonia. Ventilator-associated pneumonia remains a major healthcare burden, adding to time in intensive care, costs of hospitalization and increasing the use of antibiotics. Infections associated with mechanical ventilation can result in sepsis and remote organ damage, brain damage in neonates and delirium in elderly patients.
Ceragenins are small molecule mimics of antimicrobial peptides (peptides found natively in the body) with a large range of antibacterial and antifungal activity. A ceragenin may be used to provide antimicrobial protection to the abiotic surface of an endotracheal tube. In this study we developed a coating with covalently bound CSA-131 (a ceragenin) to coat endotracheal tubing and prevent biofilm growth. The challenge has been making a coating that will effectively kill the biofilm and successfully adhere to the tubing, while preventing the CSA-131 from eluting out of the coating.
Methodology
A hydrogel film, containing CSA-131, was generated on endotracheal tubes. Elution of CSA-131 was quantified using the drip-flow biofilm assay, antifungal and antibacterial activity was measured with repeated inoculation in growth media, biofilm formation was observed through electron microscopy.
1. Create different kinds of CSA coatings (hydrogel film) and test for efficacy and elution
a. 24, 48, and 72 hour biofilm assays with different CSA coatings
i. Inoculate with 106 or 103 PA or MRSA
ii. Incubate for 24, 48, or 72 hours
iii. Sonicate tubing to remove biofilm, serially dilute, and plate
iv. Measure colony growth and compare to controls
b. Drip-flow biofilm assay
i. Run 10% TSB/PBS solution through drip-flow for various time intervals
ii. Run 40 mL of 103 PA or MRSA through drip-flow at various time intervals
iii. Test tubing for biofilm by sonicating, serially diluting, plating and counting colonies
c. Observe biofilm formation with electron microscopy.
Results and Discussion
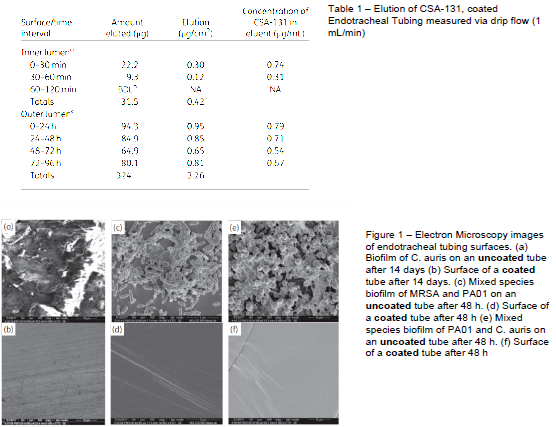
Coatings containing CSA-131 showed controlled elution of CSA-131, with concentrations released of less than 1 μg/mL (see Table 1), which is what we were hoping for. The eluting CSA prevented fungal and bacterial growth of coated endotracheal tubes for extended periods, while uncoated tubes were colonized by bacteria and fungi. In Figure 1 we see the drastic difference between the CSA coated and non-coated endotracheal tubing. There is significant biofilm growth on the uncoated tubing, while we observe an absence of biofilm growth on the CSA coated tubing.
Conclusion
Thin coatings containing CSA-131 provide protection against bacterial or fungal colonization of endotracheal tubes. This protection prevents fungal and bacterial biofilm formation on the tubes and reduces endotoxin associated with tubes. This coating could work well to decrease the infection and inflammation that has been caused by intubation in the past.