Steven Passey and Dr. Alonzo Cook, Chemical Engineering Department
Introduction:
According to the National Kidney Foundation, 26 million American adults have kidney disease, 47,000 Americans died from kidney disease in 2013, and over 100,000 people await kidney transplants. Current treatment of kidney failure includes dialysis and kidney transplant. Unfortunately, those undergoing dialysis may experience fatigue and other complications. In addition, there is a vast shortage of available kidneys for transplant.
The eventual goal is to use self-organizing iPSC to produce all needed cell types on a prepared protein scaffold to form a functioning kidney. While there are many engineering problems to solve, research into iPSC differentiation is an essential starting place.
Verification of the kidney progenitor cells is the first step to production of all needed kidney cell types. Although there are about 30 kidney cell types, renal progenitors could proliferate and differentiate into all needed components that exist in the human kidney. Current researchers use FGF9 or FGF2 and CHIR0021 to produce the above listed renal progenitors; however, a conditioned media approach could be substantially more simple and less expensive.
Methodology:
Renal Cortical Tubular Epithelial (RCTE) cells were cultured in a solution including DMEM, Fetal Bovine Serum, Amphotericin b, Penicillin and Streptomycin. Conditioned growth media was collected and saved. IPSCs were cultured in a solution of Essential 8 media on Vitronectin-coated chamber slides.
Trials were performed including 9, 6, and 3-day exposure periods to 75%, 50%, and 25% RCTE conditioned media. A negative control of 100% Essential 8 Media accompanied each exposure period. RCTE cells cultured in RCTE growth media serve as positive controls for each exposure period.
Results:
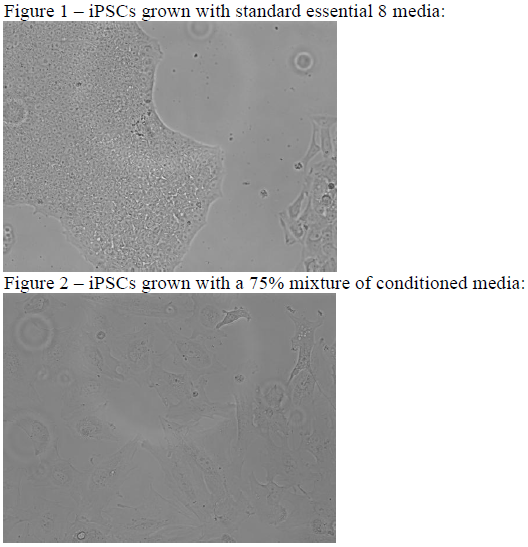
Images of cells were taken every day. Control cell colonies were compared against experimental with the hypothesis that morphological differences would be visible at the cellular level. As can be seen in Figure 1, the control iPSCs are uniformly circular and of the same size. In Figure 2, iPSCs treated with conditioned media display spindle extensions and variability in plasma membrane. We also ran a pair of blotting assays (results not shown), which were unsuccessful due to complications with the antibodies against the protein aquaporin1. However, we were able to present a poster and oral presentation at BYU’s Biomedical Engineering Society Conference.
Discussion:
The project of using conditioned media to initiate differentiation was a success and morphological differences were noted in our cell cultures. However, current research journals have published papers detailing the successes of other labs in creating kidney organoids. It seems as though more successful methods include the use of exposure to reagents to induce differentiation rather than conditioned media. We were able to adjust our plans by attempting a replication of this new approach, but were unsuccessful due to antibiotic-resistant bacteria in our cell media.
Conclusion:
Further research will continue in developing kidney organoids; however, the use of reagents such as CHIR and FGF9 appear to be more effective than the conditioned media of RCTE cells. Notwithstanding, it should be noted that the most successful models for producing kidney organoids maintain the use of feeder layers for the iPSCs during the differentiation process. A combination of feeder cell layers and conditioned media is still an appealing field for further study.