PI: Jeffery S. Tessem
Evaluation of academic objectives
The academic objectives of this Mentoring Environment Grant (MEG) were to 1) train undergraduate students in the scientific process to prepare them in careers as physicians and scientists, and 2) to examine the relationship between in utero Nr4a1 beta cell specific deletion during maternal overnutrition and obesity on beta cell mass at birth and diabetic onset. We were successful in accomplishing these objectives, as evidenced by the training of four undergraduate students, presentation of research and local and national meetings, and initial preparation of a manuscript reporting our findings.
Articles in preparation resulting from this MEG
Draney, C. et al. In utero beta cell deletion of Nr4a1 with maternal high fat feeding results in embryonic lethality. In preparation.
Evaluation of the mentoring environment
MEG group meetings-The four trainee students and I met once a week for 30 minutes (in addition to the overall lab meeting). In this meeting the students presented data, discussed results and experimental improvements, and planned the next week’s set of experiments. In addition the students presented papers dealing with the project during each semester in our overall group meeting.
Experimental design, data acquisition and data analysis-The students were coached in experimental design, data acquisition and data analysis. The students then met with me and we went through their interpretation and design. Together we made modifications to make the science and experimental interpretation sound.
Technique mastery-Each student became proficient in a variety of techniques (RTPCR, Western blotting, Insulin secretion assays, etc.). I trained each student in the techniques, and in addition each student had a particular technique that they mastered and improved on by training other students in the MEG group and in the lab.
Scientific writing and manuscript preparation-These students worked very closely with me for the writing of local and national abstracts, ORCA applications, other submitted papers, and the paper that is directly linked to this research. These writing session consisted of initial drafts, edits and rewriting. The students improved significantly in their scientific writing through the MEG.
Students Mentored
- Amanda Hobson-pancreatic histology and immunohistochemistry. Amanda graduated in April 2016 and is currently a Master of Public Health student in the Health Science department. She completed her time in my lab as the author on two papers (one as a first author), as well as two others that are currently in preparation. She also presented her data at the 2015 and 2015 UCUR and at the 2015 EB.
- Carrie Draney-animal breeding, glucose tolerance and insulin tolerance tests. Carrie graduated in April 2015 with a B.S. in Dietetics. She continued in my lab as a M.S. student in Nutritional Science. She defended her thesis in December 2016. Carrie will complete her time in my lab as the author on three papers (one as the first author), as well as four other that are currently in preparation. She presented her data at the 2015 UCUR and at the 2016 EB.
- Brent Walker-insulin secretion assays and blood glucose measurements. Brent will graduate in December 2016 with a B.S. in nutritional science. Brent has been accepted to a number of medical schools, and will be attending Rocky Vista University College of Osteopathic Medicine. Brent left the lab as an author on one paper. In addition, Brent presented his data at the 2015 and 2016 UCUR and at the 2016 EB.
- Jason Ray-RNA and protein analysis. Jason is currently applying for Ph.D. programs in Biochemistry, is a finalist for the Hertz Foundation Fellowship, and will graduate with his B.S. in April 2017 in Chemistry. Jason is the author on two papers (one as a first author), as well as three more that are currently under preparation.
Below are lists of papers published and posters presented at scientific meetings, which have resulted from mentoring in my lab since this MEG grant was awarded in 2014. BYU student authors are in bold to emphasize the number of students who have benefited from mentoring in the Tessem Lab.
Publications
- Hobson A, Draney C, Stratford A, Becker TC, Lu D, Arlotto M, Tessem JS. Aurora Kinase A is critical for the Nkx6.1 mediated β-cell proliferation pathway. Islets. 2015 Jan 2;7(1):e1027854. doi: 10.1080/19382014.2015.1027854. Epub 2015 Jun 1. PMID: 26030060
- Draney C, Hobson AE, Grover SG, Jack BO, and Tessem JS. Cdk5r1 Overexpression Induces Primary beta-Cell Proliferation. Journal of diabetes research 2016: 6375804, 2016.
- Ray JD, Kener KB, Bitner BF, Wright BJ, Ballard MS, Barrett EJ, Hill JT, Moss LG, and Tessem JS. Nkx6.1-mediated insulin secretion and beta-cell proliferation is dependent on upregulation of c-Fos. FEBS letters 590: 1791- 1803, 2016.
- Reynolds MS, Hancock CR, Ray JD, Kener KB, Draney C, Garland K, Hardman J, Bikman BT, and Tessem JS. Beta-cell deletion of Nr4a1 and Nr4a3 nuclear receptors impedes mitochondrial respiration and insulin secretion. American journal of physiology Endocrinology and metabolism ajpendo 00022 02016, 2016.
- Strat KM, Rowley TJ 4th, Smithson AT, Tessem JS, Hulver MW, Liu D, Davy BM, Davy KP, Neilson AP. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J Nutr Biochem. 2016 Sep;35:1- 21. doi: 10.1016/j.jnutbio.2015.12.008. Epub 2016 Jan 23.
Posters
- Hobson A, Draney C, Stratford A, Lu D, Becker T, Arlotto M, Tessem JS. AURKA induces primary β-cell proliferation by downregulating p53. Experimental Biology 2015, March 29-April 1, 2015. Boston, MA.
- Ray J, Bitner B, Kener K, Wright B, Tessem JS. c-Fos mediates the proliferative and insulin secretion capacity of Nkx6.1 in β-cells. Experimental Biology 2015, March 29-April 1, 2015. Boston, MA.
- Draney C, Hobson A, Tessem JS. Expression of Cdk5r1, and not Cdk5, induces primary beta cell proliferation. Experimental Biology 2015, March 29-April 1, 2015. Boston, MA.
- Reynolds MS, Hancock CR, Ray JD, Kener KB, Hardman JM, Tessem JS. β-cell Deletion of Nr4a1 and Nr4a3 Nuclear Receptors Impedes Mitochondrial Respiration and Insulin Secretion. Experimental Biology 2016, April 2016, San Diego, CA.
- Draney C, Hobson A, Tessem JS. HDAC1 increases functional β-cell mass. Experimental Biology 2016, April 2016, San Diego, CA.
- Rowley TJ, Bitner BF, Ballard M, Smithson AT, Neilson AP, Tessem JS. Monomeric cocoa procyanidins enhance functional β-cell mass. Experimental Biology 2016, April 2016, San Diego, CA.
- Wright B, Garland K, Tidwell C, Kang S, Tessem JS. Aged islets are refractory to Nkx6.1 mediated β-cell mass. Experimental Biology 2016, April 2016, San Diego, CA.
Description of the results/findings of the project
Aim 1: Does embryonic Nr4a1 beta cell specific Nr4a1 deletion with maternal overnutrition affect beta cell mass at birth?
Using β-cell specific Nr4a1 KO mice we sought to determine the effect of in utero β-cell specific Nr4a1 deletion. Mice were generated by breeding Nr4a1Flox/Flox female mice with Nr4a1Flox/Flox INS1-Cre-ER/TM male mice. This produced Nr4a1Flox/Flox and Nr4a1Flox/Flox INS1-Cre- ER/TM pups, with the later being used as the experimental population. We also breed Nr4a1Wt/Wt female mice with Nr4a1Wt/Wt INS1-Cre-ER/TM male mice, in order to produce Nr4a1Wt/Wt INS1-Cre-ER/TM mice that were used as controls. Dames were fed a high fat-HF or low fat-LF chow for 1 month prior to being paired for mating. Upon verification of pregnancy by vaginal plug, dames were separated from male, kept on their respective HF or LF feeding regime, and received either corn oil or tamoxifen injections from e11.5 until birth.
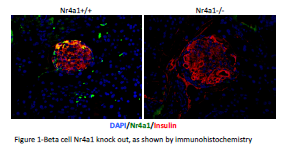
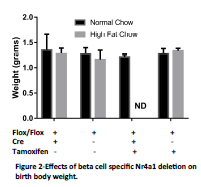
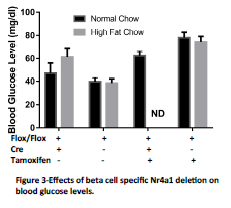
For this study mouse pups were harvested at birth to determine the effect of in utero Nr4a1 deletion in the context of maternal overnutrition. We measured this effect by measuring body weight, blood glucose and by harvesting pancreata for histological measurements of beta cell mass. We verified that Nr4a1 deletion resulted in decreased Nr4a1 expression in the pancreatic beta cell by causing Nr4a1 deletion in adult mice (Figure 1). We observed no significant difference in pup body weight at birth (Figure 2). Furthermore, no differences were observed in blood glucose levels, although there was a trend to increased blood glucose in the Tamoxifen treated animals (Figure 3). Of importance to this study, we were unable to obtain the Nr4a1Flox/Flox INS1-Cre-ER/TM mice from dames fed a high fat diet and treated with Tamoxifen. In each of these litters, the dame ate the pups soon after birth and we have been unable to obtain the necessary pups. This has occurred with 8 different litters using 7 different female mice as dames. Interesting, the only time that a litter was not eaten by the dame on high fat diet that was injected with Tamoxifen from an Nr4a1Flox/Flox x Nr4a1Flox/Flox INS1-Cre-ER/TM mating, all of the pups were Cre-, and thus had no recombination. In consultation with embryologists at the University of Colorado, this is indicative of serious and significant embryonic defects. For this reason we are preparing amendments to our study to no harvest the mice prior to parturition.
Aim 2: Does embryonic Nr4a1 beta cell specific Nr4a1 deletion with maternal overnutrition lead to diabetic onset?
We were unable to complete this Aim for two reasons. First, the inability to obtain live Nr4a1Flox/Flox INS1-Cre-ER/TM pups from high fat fed, Tamoxifen injected mice left us without the ability to make comparisons to our critical genotype. Second, we attempted to allow the animals from the other breeding schemes to age. Animals born to mothers that received the Tamoxifen injections died within 1 week of birth. This suggests that we may need to revisit the method for treating with Tamoxifen. We will test both Tamoxifen in the drinking water, allowing us to use a lower and more constant dose to induce recombination, and we may delay Tamoxifen injections until the last week prior to birth. These studies are ongoing.
Conclusions
This work has demonstrated a very interesting set of data, and has presented us with more questions to pursue. Our findings that in utero beta cell specific Nr4a1 deletion results in serious survival issues demonstrates that Nr4a1 is essential and demonstrates the importance of continuing with these studies.
Summary of Spending
The budget for this project was spent as follows:
- Animal care costs, LSB mouse facility ($6,000)
- PCR enzymes and primers for genotyping the mice ($2,000)
- Injection needles, glucose test strips and glucometer for measuring blood glucose ($3,000)
- Student ravel for meetings ($4,000)
- Student wages ($5,000)