PI: Jeff Edwards
Evaluation of academic objectives of the proposal
The objectives of this award were met, especially in regard to the mentoring focus of the award. The students have been mentored and trained in an effective manner as outlined in my Mentoring Plan (abbreviated below). This includes mentoring students in research, writing, publishing, presenting at conferences, etc. Indeed, one measure of the success of mentoring is demonstrated by the fact that all the students graduating from my lab during this time were accepted to the professional school of their choice (see students listed below). Research, especially where the students are actually involved in all aspects of it including data collection and analysis, as well as publishing can play a major role in their acceptance to professional schools and set them apart from other candidates.
Regarding the research towards funded by this award we have attended several international and regional conferences and published abstracts, with the centerpiece publication of the project anticipated to be submitted later this year, as well as 2 other manuscripts related to the project submitted and one published. There were many student co-authors on these manuscripts and abstracts.
Student research presentations given include at the international Society for Neuroscience meeting in Washington, DC and Chicago, Illinois; the Intermountain Society for Neuroscience Symposium, and Utah Conference on Undergraduate Research. Data was also used to submit three NIH grant proposals.
Overall, we have made excellent progress with the time and money that we have had to work with on this project, and in student mentoring. I am happy with the progress, which in no way could have happened without this MEG award to provide the needed supplies and student support over the last two years. We have really provided a successful and meaningful mentoring experience for the students involved, and at the same time produced a lot of meaningful and compelling data related to reward and addiction.
Evaluation of the Mentoring Environment
The following is my philosophy of mentoring: 1) Teach the fundamental elements of hypothesis driven research. 2) Allow students to explore their own interests within the realm of the laboratory umbrella. 3) Provide students with the time, personal supervision and overall training they need to be successful individuals in the lab and in their futures, professionally and academically. 4) Use laboratory meetings, journal clubs, attendance at professional meetings, presenting posters as an essential way to train students to develop critical analyzing and thinking skills as well as oral presentation skills. 5) Encourage student development through grant writing, training experiences by allowing students to teach each other and collaborate through group work. 6) Encourage positive interactions with myself and others in the lab. 7) Prepare students with the professional skills required beyond the undergraduate degree in their schooling and professions by accomplishing the aforementioned aims.
To help accomplish this general goal of mentoring as listed above I have several evaluation endpoints for the students to accomplish and tools to do it. 1) Initially, I use an undergraduate training program as a tool to teach students. All undergraduates go through a training program before they can enter the lab to perform experiments. The training is a collaborative effort between my lab and several others with the overall goal of providing consistent, general laboratory training to all new students. This training includes safety, animal handling, and a variety of laboratory skills. All of this is done under the supervision of advanced undergraduate and graduate students as well as myself. 2) New students entering the lab also receive 3-5 hours of background training together with me the first month they are in the lab. This background training is designed to update their basic understanding of learning and memory as well as reward/addiction science and to get them excited about the research by explaining what the experiments are and what they will be doing in the lab and why. 3) All students are expected to acquire good, publishable data. This is ensured by weekly lab meetings where the students present their data and we review it together. I also watch students in the lab doing the experiments at least weekly to ensure the experiments are performed properly, that they are learning from the experience, and that they have a better mentored experience than undergraduates who are left to work on their own with little or no guidance. 4) They must learn to present, not just their data, but scientific information to others. Initially this is practiced at laboratory meetings with their own data, then students present at journal clubs and finally at conferences. Through this process they learn good oral communication skills, how to present data to others as well as get to know and discuss science with researchers from around the world. I had three graduate students and 8 undergraduates travel to different meetings to present their data in the last two years (department funds also helped with the travel costs for this many students). 5) I have my students apply for internal competitive research grants and my graduate students for NIH pre-doctoral grants. Several undergraduates were awarded ORCA grants in 2015 and 2016. This has given them the opportunity to research different project ideas, search scientific databases and write their own grants with minor editing help from me. I attempt to make this process as independent as possible, yet guided, providing an overview for how to write a successful grant application. 6) I want the students to publish. While I discussed this above I think it worth mentioning that I have many undergraduates who will be co-authors on manuscripts.
The research experience students received in my lab was a key for most getting acceptance into professional school. All my students who have finished have been accepted into the graduate or professional school of their choice or are still applying (see section III below). In conclusion, the students in my lab are getting excellent mentoring and training as illustrated by the tangible products of research listed in section IV below. This experience is going to be useful in their future and in getting excepted and succeeding in the professional school of their choice, which I think is one of the main overall purposes of the MEG award while establishing lasting and meaningful relationships with myself and others in the lab.
List of students who participated
Profile of Undergraduate Students Previously Trained (2015–2016) Collectively, 100% of the students completing training in my lab in the last 2 years were accepted to the professional school of their choice or are currently Appling.
Grad., Student Name, Current Status (School)
April 15, Sam Lo, Med school, applying
Apr 14, Zach Hopkins, Med school, Univ. of Utah
April 15, Jaden Willard, Dental school, applying
Apr 14, Brittany Harrison, Applying, grad school
April 16, Jorden Barrow, Med school, applying
Apr 14, Bethany Walker, Grad school, UCF
April 16, Tyler Hammond, MD/Phd, Univ of Kentucky
Apr 14, Dane Lyman, Med, UCF
April 16, Cole Romney, Med school, applying
Apr 14, Nathan Christensen Grad school, Touro Univ.
April 16, Dan Hall, Med school, Univ. Wash.
Apr 14, Bradley Prince, Med school, Univ. of Calgary
Aug 14, Sarah Davis, Pharmacy, Ohio State Univ.
Apr 15, Deson Haynie, MPH; John’s Hopkins
Jun 14, Cannon Nelson, Med school; applying
All students were involved in one of 2 groups, with some doing electrophysiology and others doing molecular biology. These students represent those who were mentored in my lab and acquired the data used for the grant applications, abstracts, publications and presentations listed above. Again, they also collectively were awarded several ORCA grants. The main graduate student working on this project was also awarded a graduate fellowship awards for their work. I have had some fantastic undergraduates and particularly graduate student, Teresa Nufer who have done a great job with these projects.
Description of results/findings
Again, we have published one manuscript, have another two others submitted from funding from this MEG grant. We also have published many abstracts, presented our data at regional and international meetings, and used this data for three NIH grant proposals. This MEG provided money that went towards data that contributed to attaining one NIH grant in 2016. It would have been impossible to acquire the data to get this grant without the MEG funding. Finally, the major manuscript supported by this award we anticipate will be submitted late this year.
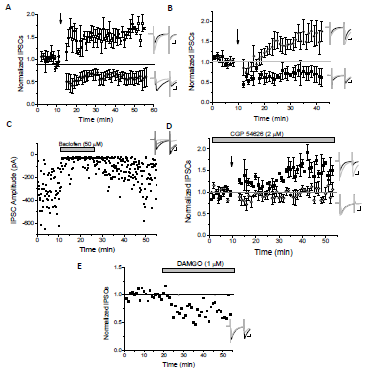
Our goal was to investigate the role of GABA cells in the ventral tegmental area, which is the reward center of the brain, and how drugs of abuse alter their synaptic activity potentially leading to addiction. In order to understand and treat addiction it was necessary to understand how the VTA is regulated at the synaptic level. We therefore examined how inhibitory inputs to VTA inhibitory cells are regulated and specifically how synapses onto these cells can be modified, as these modifications, known as synaptic plasticity, are what lead to the long-term addictive effects of drugs versus the immediate reward. Surprisingly, we identified that there are actually two different types of plasticity onto VTA GABA cells. One population of GABA cells undergo potentiation of inhibitory inputs and another cohort undergo depression of these inputs. We worked to determine that those potentiating are mediate by an excitatory receptor, NMDA, and that those undergoing depression are mediated by GABAB receptors (see figure 1 below). However, in order to discriminate between the two we decided to employ optogenetics to determine if differences in plasticity were input specific. Optogenetics is a way to activated individual pathway inputs from different brain regions to VTA GABA neurons using light. Currently, we have an animal model and equipment needed and are performing these experiments (see figure 2 below). These are additional experiments beyond what we initially proposed, but it is the important salient question now to ask before we publish our data. Lastly, once these are characterized we will examine how drugs of abuse, morphine and cocaine alter this plasticity. I currently have a DEA license and we have some ongoing experiments using drugs of abuse. April 16 Tyler Hammond MD/Phd, Univ of Kentucky Apr 14 Dane Lyman Med, UCF April 16 Cole Romney Med school, applying Apr 14 Nathan Christensen Grad school, Touro Univ. April 16 Dan Hall Med school, Univ. Wash. Apr 14 Bradley Prince Med school, Univ. of Calgary Aug 14 Sarah Davis Pharmacy, Ohio State Univ. Apr 15 Deson Haynie MPH; John’s Hopkins Jun 14 Cannon Nelson Med school; applying Data for published projects can be found as a pdf file using PubMed to search for the following references.
Data in these publications was acquired using the MEG awards:
Publications:
Merrill CB, Friend LN, Newton ST, Hopkins ZH, Edwards JG. 2015. Ventral tegmental area dopamine and GABA neurons: Physiological properties and expression of mRNA for endocannabinoid biosynthetic enzymes and type I metabotropic glutamate receptors. Scientific Reports. Nov 10;5:16176. doi: 10.1038/srep16176. (Impact Factor: 5.58).
Lindsey Friend, Jared Weed, Philip Sandoval, Jeffrey G. Edwards. CB1-dependent LTD in Ventral Tegmental Area GABA Neurons: a Novel Target for Marijuana. Submitted (Journal of Neuroscience).
Katrina Hurst, Corinne Badgley, Tanner Ellsworth, Spencer Bell, Lindsey Friend, Brad Prince, Jacob Welch, Zack Cowan, Ryan Williamson, Chris Lyon, Brian Poole, Michael Christensen, Jarrod Call and Jeffrey G. Edwards. The Putative Cannabinoid Receptor GPR55 Modulates Hippocampal Synaptic Plasticity. Submitted (Hippocampus).
Data
Figure 1. LTP/LTD of GABA inputs to VTA GABA cells. A) 5Hz stimulation (arrow) elicits either LTP or LTD in ALL control cells (n=4 each). B) CB1 KO mice (n=5 LTP, n=4 LTD) still exhibit LTD and LTP (though initial LTP is slightly depressed). C) Example of GABAB agonist baclofen depressing IPSCs and increasing failure rates. D) GABAB antagonist blocks LTD cells (n=4), but not LTP cells (n=2). E) μ opiate receptor agonist DAMGO (1 μM) induces IPSC depression in this example. Traces just before (black) and after drug application (light gray); Scale bars: 10ms, 50pA.
Figure 2. Optogenetics. Blue light-evoked GABA LTP of IPSCs in a VTA DA cell before (black) and after (gray) conditioning. This demonstrates our current ability to induce plasticity in optogenetic slice model. Scale bar; 10ms, 50pA
These few pieces of data demonstrate the major part of our find that ventral tegmental area GABA neurons exhibit a synaptic plasticity known as LTP and LTD that is mediated by NMDA and GABAB receptors, respectively.
Description of how the budget was spent
The following is a list of approximately how much in each major category was spent using the MEG funds awarded.