S-glutathionylation of VMAT by Acute Methamphetamine
Spencer McCarthy swynnmac@gmail.com smccarth
Scott Steffensen Psychology/Neuroscience
Introduction
Methamphetamine (METH) has long been regarded as a potent addictive drug and
psychostimulant. The addictive effects can be localized to the VTA, specifically activity of
dopaminergic neurons in the nucleus accumbens (NAc). Overactivity of these neurons results in
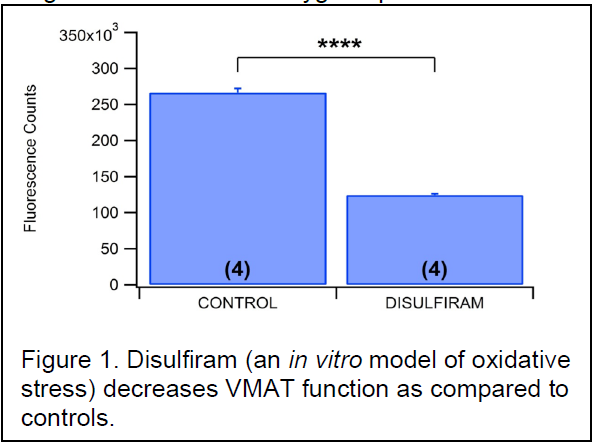
the pleasurable and addictive of METH. Various theories have been proposed as to the mechanism of this excess dopamine release; including a role for reactive oxygen species. In a recent publication, our lab has already demonstrated that METH induces the formation of reactive oxygen species (ROS) in the axon terminals of dopaminergic neurons in the nucleus accumbens and that blocking the formation of these ROS also attenuates METH’s effects on dopamine release. Using disulfiram as a controlled ROS, we have shown that oxidative stress significantly reduces the function of the vesicular monoamine transporter (VMAT) (Fig. 1). The purpose of the proposed experiment was to demonstrate the direct effects of METH
on the VMAT2, particularly on reactive residues. We hypothesized that the ROS inhibit the
function of the vesicular monoamine transporter 2 (VMAT2) by oxidizing the cysteine-488 residue.
Methodology
Results
Animals were divided randomly into three groups that were then doubleinjected
with saline/saline, saline/METH (10 mg/kg), and BD 1063 (30 mg/kg) / METH (10 mg/kg), respectively. The injections were approximately 10 minutes apart. 30 minutes after the second injection,
NAc tissue was immediately harvested and chilled. The tissue samples were lysed in ice-cold TNTE
buffer (containing the following in mM: 50 Tris pH 7.4, 150 NaCl, and 1 EDTA in addition to 0.5% Triton X-100) for 10 min with a rotation at 4°C. Tissue lysates were triterated 10 times using a 23 gauge needle and cleared by centrifugation at 14800 G for 10 min. Anti-VMAT2 antibody (EMD Millipore, Billerica, MA) was coupled to magnetic dynabeads (ThermoFisher, Waltham, MA) and immunoprecipitated VMAT2 from tissue lysates according to the manufacturer’s instructions. Immunoprecipitated VMAT2 was run on a 10% SDS-PAGE and immunoblotted for anti-GSH (Virogen, Watertown, MA) and anti-VMAT2 antibodies. Animals were randomly divided into three groups that were then double-injected with
saline/saline, saline/METH (5 mg/kg), and BD 1063 (10 mg/kg) / METH (5 mg/kg), respectively.
Injections were approximately 10 minutes apart. 30 minutes after the second injection. The
animals were then perfused as previously described (Jang et al., 2015). Briefly, mouse brains
were removed under pentobarbital anesthesia (80 mg/kg, IP), post-fixed with 10% sucrose/4%
paraformaldehyde for 2 hr and cryoprotected in 30% sucrose for a least 48 hr and cryosectioned
into 30 μm thick slices. Brain tissues were incubated in blocking solution containing 4% normal
goat serum and 0.1% bovine serum albumin at room temperature for 1 hr. After rinsing in 0.01 M
PBS, the tissues were incubated with primary antibody for anti-mouse 8-hydroxyguanosine (8-
OHG; a cellular marker of oxidative damage; 1:400, Abcam, MA, USA) overnight at 4 °C. The
sections were then processed with secondary antibody, goat anti-mouse Alexa Fluor 594 (red;
1:250). All sections were cover-slipped with mounting medium (Vector Laboratories, Burlingame,
CA, USA) and were imaged with a 20X objective using a Carl Zeiss AxioCam digital camera
attached to the microscope (Carl Zeiss Axioskop, Oberkochen, Germany). The fluorescence
intensities (FI) of 8-OHG in sections (Area: 30,000 μm2) were estimated by computerized
densitometry (ZEN, Oberkochen, Germany).
Discussion
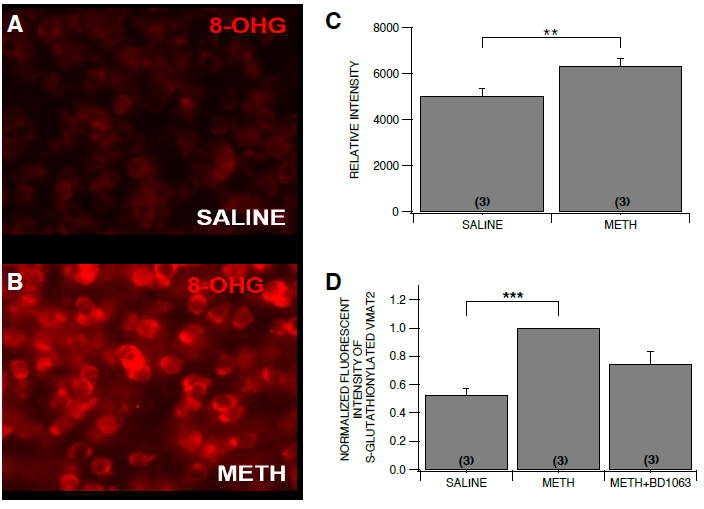
Our results confirm our model of dopamine release in the NAc. Increased fluorescent counts of
oxidative stress using 8-OHG as a marker indicate that METH induces the formation of ROS.
Additionally, the increased count of glutathionylated VMAT2 cysteine residues supports the
hypothesis that the METH-induced oxidative stress results in the S-glutathionylation of VMAT2.
Blocking upstream causal factors of the formation of ROS’s using BD1063 gives additional
credence to the idea that if oxidative species are not formed, glutathionylation events will not take
place on VMAT2.
The implications of this project help pave the way for future experiments involving VMAT2 and
dopamine release. Given that the glutathionylation of VMAT2 results in a decrease in VMAT2
activity, dopamine would be less likely to be packaged into vesicles leading to increased
concentrations of cytosolic dopamine in the axon terminal. We believe this increased
concentration gradient reverses the dopamine transporter (DAT), and the intracellular dopamine
is released into the synapse. This increased perisynaptic dopamine results in the pleasurable
and addictive effects of METH.
Conclusion
This project ties together the cellular events caused by methamphetamine and the synaptic
release of dopamine in the nucleus accumbens. Methamphetamine-induced formation of radical
oxygen species had been well established by the Steffensen lab, but the link between the ROS
and VMAT2 activity had yet to be established. Using immunohistochemistry, we confirmed this
link and established METH-mediated basal release in the ventral striatum. Our results help
clarify some of the mechanisms underlying meth addiction in hopes of eventually overcoming the
devastating effects of the disease.