Keaton Karlinsey and Alonzo Cook, Department of Chemical Engineering
Introduction
The purpose of this project is to develop an effective treatment for damaged nerves. A team led by Dr. Cook has been working in this area for several years. Their project used a combination of biochemical treatments on rats with damaged sciatic nerves to improve the rate and quality of regeneration. The team is comparing the recovery of the nerves through a treatment receiving a combination of Lysophosphatidylcholine (LPC) and Nerve Growth Factor (NGF) with a treatment receiving only NGF and with a control that receives no treatment. LPC has been shown to upregulate the trkA receptor for NGF, thus increasing the effectiveness of NGF treatments. The results of this study showed no significant difference in the rate of regeneration over 6 weeks.
This new project expands upon the results and conclusions of Dr. Cook’s current project. Dr. Cook’s project only applied each treatment once, but this project uses multiple treatments of NGF to maximize nerve recovery. Instead of giving a single application of NGF 7 days after the crush injury, when the trkA receptor is at its peak, treatments are given 5, 7, and 9 days post-injury in order to provide a greater effect from NGF. In order to avoid further trauma to the rat and minimize potential recovery inhibitors, we designed a device, described below, that allows multiple NGF injections to be administered directly into the nerve without performing extra surgeries to expose the nerve. The results from this multiple injections study will be compared to Dr. Cook’s earlier results to determine the effectiveness of an NGF-LPC combination treatment.
Materials and Methods
To simulate traumatic injuries, a crush was performed on the left sciatic nerve of female Wistar rats. There were a total of eight experimental groups ( n=9, 72 rats total), organized as follows: Crush, Crush+LPC, Crush+LPC+NGF (with 1, 2, or 3 NGF injections), and Crush+NGF (with 1, 2, or 3 NGF injections). For rats receiving LPC, an injection of 1% wt./vol. egg-derived lysophosphatidylcholine (Sigma-Aldrich, St. Louis, MO) was performed immediately after the crush injury. Rats that did not receive a treatment of LPC were injected with phosphate-buffered saline (PBS). NGF injections were performed 5, 7, and 9 days post-injury, using 80 ng/mL human-derived NGF-β (Sigma-Aldrich). All rats that received fewer than 3 NGF injections were injected with PBS as a control.
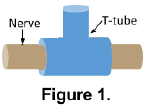
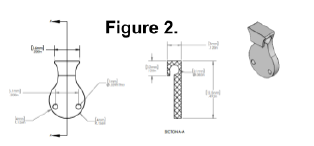
To avoid trauma to the rats and potential surgical complications, we designed a nerve guide device that could be used to perform injections directly into the nerve following surgery without re-exposing the nerve through a second surgery. In the initial proposal for the project, a silastic T-shaped conduit (a T-tube, see Figure 1) that wrapped around the nerve was described, but preliminary tests using this design revealed it to be impractical and unreliable. Eventually, we designed another device (BYU Precision Machine Laboratory, Provo, UT), shown in Figure 2, that encompassed the damaged nerve, with a “tab” leading up to the surface of the skin where it could be palpated. The device was inserted immediately following the crush injury and initial injection, and NGF or PBS injections were performed by palpating the top of the device, inserting a needle into the skin at the site of the device, and using the broad portion of the device to guide the needle into the channel containing the nerve.
Recovery of the rats is measured electrophysiologically, functionally, and histologically. Electrophysiological measurements are made by inserting stimulating and reference electrodes into the nerve and surrounding tissue, stimulating the nerve, and measuring the compound muscle action potential (CMAP) voltage across the injury. Data from the injured nerve is compared to data recorded on the uninjured side of the body and expressed as a percentage recovered in relation to the healthy nerve. Functional recovery is measured through the Basso, Beattie, and Bresnahan (BBB) scale, which objectively breaks down the rats’ gait on a scale from 0-21, as well as a toe-spread test. In the toe-spread test, rats’ feet are covered in ink prior to walking across a papered surface, and the distance between toes on the footprints is measured. The spread between toes in the injured foot is an indication of how much control the rat has of its toes while walking and can be used to measure functional recovery. Histology is performed with a myelin stain, which allows us to assess the total recovery of nerve fibers by number, size, and degree of remyelination (G-ratio).
Results and Conclusions
Due to the changes that were required in designing and manufacturing the nerve guides, data collection on the project was delayed by several months. As of December 2016, we are still collecting data on the rats, and we plan to finish collecting all the data by April-May 2017. However, preliminary electrophysiological data suggests that both groups receiving 3 treatments of NGF are recovering substantially more nerve conductance than all other groups, though our sample sizes are currently too small to obtain any significant statistical measure of confidence. It is interesting to note that the LPC+3NGF group showed slightly less recovery than the PBS+3NGF group, though both groups showed far greater recovery than any other experimental group measured. While the data are preliminary and no real conclusions can be drawn, it is noteworthy that we found a difference between the two groups receiving 3 NGF injections and all other experimental groups in this study.