Kevin Garland and Jeffery Tessem, Department of Nutrition, Dietetics & Food Science
In 2010, diabetes was the 7th leading cause of death in America, and as of 2014, 9% of the world’s adult population was affected by it. In both Type I (T1D) and Type II diabetes (T2D), a loss of β-cell mass and overall function is observed. β-cells essentially stop proliferating after adolescence, therefore developing mechanisms to increase function and/or induce proliferation in β-cells could serve as a powerful treatment and even a cure for both T1D and T2D. Overexpression of the β-cell transcription factor Nkx6.1 induces β-cell proliferation (1) and up-regulates expression of the nuclear receptor Nr4a1 (2). Nr4a1 has been shown to be necessary and sufficient for Nkx6.1-mediated β-cell proliferation (2). In addition to its proliferative effects, we also know that Nkx6.1 is necessary for maintaining proper β-cell function (3). To further investigate the mechanisms of β-cell proliferation and maintenance, we bred mice that lacked the Nr4a1 gene, anticipating that these animals would exhibit increased blood glucose levels under normal conditions, as well as during a glucose tolerance test. We also examined relative immunity cell levels in the knockout animals.
An Nr4a1 knockout animal was obtained from Dr. Lily Chao at USC. This was bred with wild type animals, whose offspring were bred with each other to eventually obtain a colony of homozygous knockout animals. Experiments were done with four different groups of animals: wild types fed normal chow, wild types fed with high fat chow, knockouts fed with normal chow, and knockouts fed with high fat chow (D12492, Research Diets Inc.). High fat chow was used because prior research indicated that a knockout of the Nr4a1 gene only affects β-cell function when the subject is also on a high fat diet. Blood was taken from the tail vein and glucose levels were measured with a Bayer Contour glucometer. Glucose tolerance tests were performed on each of the four groups at 8 weeks, 12 weeks, 16 weeks, and 20 weeks of age by injecting glucose in the peritoneal area of the animal, with the amount of glucose being proportional to the weight of the animal. Blood glucose measurements were then taken 15 min., 30 min., 60 min., and 120 min. post-injection. We also had an offer from Dr. Weber’s lab to measure levels of immunity cells from spleens and lymph nodes from our animals. These levels were counted by immunophenotyping, where cells are labelled with an antibody specific to that kind of cell, which are then counted via flow cytometry.
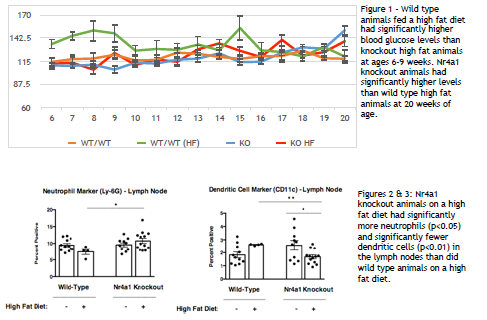
Normal blood glucose measurements were significantly higher for wild type high fat animals than for knockout high fat animals between 6 and 9 weeks of age. They were significantly higher for knockout high fat animals than for wild type high fat animals at 20 weeks of age (fig. 1). No significant differences were found between wild type and knockout animal glucose tolerance tests at any of the time points measured. In the lymph nodes, knockout high fat chow animals had significantly fewer dendritic cells (fig. 2). and significantly more neutrophils (fig. 3) in the lymph nodes than did wild type high fat chow animals.
We expected to see knockout animals to have higher blood glucose levels than wild type animals under normal conditions as well as during a glucose tolerance tests. Because we did see significantly higher glucose levels at 20 weeks of age, it could be that it takes more time for β-cell impairment to develop in knockout animals. Future experiments could be done to investigate glucose levels in Nr4a1 knockout animals at ages greater than 20 weeks.
Knockout animals were observed to have more neutrophils (immunity cells involved in the inflammation response) in their lymph nodes than were wild type animals. Increased levels of neutrophils have been shown to be a predictor of T2D, suggesting that lacking Nr4a1 may indirectly influence the likelihood of diabetes. In addition, a disruption of certain dendritic cell (immunity cells that present antigens to other cells of the immune system) functions has been shown to have implications for T1D. Knockout animals had significantly fewer dendritic cells in the lymph nodes than did wild type animals, again implicating Nr4a1 in the development of diabetes.
Although the evidence is not as strong as we were expecting, we have demonstrated that Nr4a1 could potentially have ties to β-cell function and diabetes in older animals and/or through ties to cells of the immune system, such as neutrophils and dendritic cells. It is not understood through what mechanisms Nr4a1 is tied to these immune cells, so future investigations into these relationships would be worthwhile.