Ray, Jason
CEBP/α increases functional β-cell mass
Faculty Mentor: Jeffery Tessem, Department of NDFS
Introduction
Type 1 and Type 2 diabetes are major global health concerns. Diabetes is characterized by impaired management of glucose in the bloodstream, which is due to decreased pancreatic functional β-cell mass. Functional β-cell mass is determined from the insulin secretion rate and the number of β-cells, which is determined from cellular proliferation and death rates. Increasing functional β-cell mass could cure diabetes either by enhancing pancreatic islets prior to transplantation, or by strengthening endogenous cells. Although β-cells stop proliferating around adolescence, β-cell proliferation resumes during conditions such as pregnancy and obesity (1), demonstrating that the molecular pathways necessary for replication remain intact.
The transcription factor Nkx6.1 increases functional β-cell mass by increasing glucose stimulated insulin secretion (GSIS), proliferation, and cell survival (2). Our group has demonstrated that c-Fos, another transcription factor, is a key downstream target of Nkx6.1 (3). In this study, we show that the transcription factor CEBP/α is a key intermediary between Nkx6.1 and c-Fos. We further show that CEBP/α increases functional β-cell mass in the same way as Nkx6.1.
Results
Nkx6.1 induces expression of CEBP/α
Primary rat islets were harvested and treated with adenoviruses expressing GFP or Nkx6.1. Islets were then harvested at 24, 48, and 96 hours. Pathway analysis on the microarray data shows that Nkx6.1 induces CEBP/α, and suggests that CEBP/α induces c-Fos (Figure 1A). RT-PCR of INS-1 832/13 cells treated with no treatment, adenovirus expressing GFP, and Nkx6.1 confirms that Nkx6.1 overexpression leads to increased CEBP/α expression at 24 and 48 hours (Figure 1B).
Overexpression of CEBP/α induces c-Fos
INS-1 832/13 cells were treated with no treatment or adenovirus overexpressing GFP or CEBP/α, then harvested at 24 hours. Quantitative RT-PCR analysis of samples shows that overexpression of CEBP/α induces expression of c-Fos (Figure 2A). The experiment was repeated along an 18-hour time course, showing that c-Fos is induced briefly 2 hours after overexpression of CEBP/α, and again at 18 hours after overexpression (Figure 2B).
Overexpression of CEBP/α leads to β-cell proliferation
Primary rat islets were treated with no treatment, GFP, or CEBP/α, and were tested for incorporation of 3H-labeled Thymidine. Islets overexpressing CEBP/α showed increased incorporation of 3H-labeled Thymidine (Figure 3A). INS-1 832/13 cells were treated with no treatment, GFP, or CEBP/α. 72 hours after addition of virus, cells were counted using a hemocytometer. Cells treated with CEBP/α showed increased proliferation levels over untreated cells (Figure 3B).
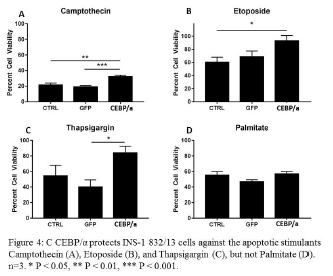
Overexpression of CEBP/α protects β-cells from various apoptotic stimulants
INS-1 832/13 cells were treated with no treatment, GFP, or CEBP/α. After 48 hours the apoptotic stimulants camptothecin, etoposide, thapsigargin, or palmitate were added. 18 hours after addition of apoptotic stimulants cells were counted with a hemocytometer. Cell counts were normalized to a fifth, untreated plate of cells in order to account for differences due to increased proliferation from CEBP/α. Results show that CEBP/α protects β-cells from camptothecin, etoposide, and thapsigargin, but not palmitate (Figure 4).
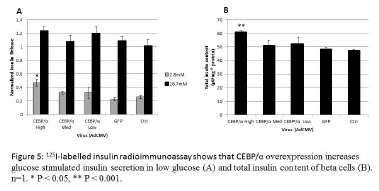
Overexpression of CEBP/α leads to increased glucose stimulated insulin secretion
INS-1 832/13 cells treated with varying concentrations of CEBP/α were tested for glucose stimulated insulin secretion using 125I-labelled insulin radioimmunoassay. The assay showed that CEBP/α increased the basal insulin secretion rate (Figure 5A) and increased total insulin content of β-cells (Figure 5B).
Methods
Cell culture, cell count assays, RT-PCR, and radioimmunoassays were all performed as previously described (3).
Conclusions
Nkx6.1 has been shown to increase functional β-cell mass by increasing GSIS, proliferation, and survival in β-cells. We have previously shown that c-Fos is an important downstream target of Nkx6.1, and that Nkx6.1 is unable to increase functional β-cell mass without c-Fos. Here, we identify CEBP/α as a key intermediary between Nkx6.1 and c-Fos.
Because of its place in the Nkx6.1-mediated pathway of increasing functional β-cell mass, and because of its effects on β-cells, CEBP/α is a potential target for therapeutics seeking to treat diabetes. These therapeutics could use CEBP/α to enhance β-cells prior to pancreatic islet transplants or to increase endogenous β-cells in diabetic individuals.
Additional studies to confirm the role of CEPB/α in the Nkx6.1 β-cell pathway are still being performed. Before submitting this study for review and publication, we will use western blots to confirm that increased CEBP/α RNA levels identified by RT-PCR correspond to increased cellular protein levels. Furthermore, we will perform at least two more radioimmunoassays with INS-1 832/13 cells overexpressing CEBP/α so that we can report our results with n = 3 in all experiments.
Works Cited
1. Butler, A. E.; Cao-Minh, L.; Galasso, R.; Rizza, R. A.; Corradin, A.; Cobelli, C.; Butler, P. C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010, 53, 2167-2176.
2. Schisler, J. C.; Fueger, P. T.; Babu, D. A.; Hohmeier, H. E.; Tessem, J. S.; Lu, D.; Becker, T. C.; Naziruddin, B.; Levy, M.; Mirmira, R. G.; Newgard, C. B. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol. Cell. Biol. 2008, 28, 3465-3476.
3. Ray, J. D.; Kener, K. B.; Bitner, B. F.; Wright, B. J.; Ballard, M. S.; Barrett, E. J.; Hill, J. T.; Moss, L. G.; Tessem, J. S. Nkx6.1-mediated insulin secretion and beta-cell proliferation is dependent on upregulation of c-Fos. FEBS Lett. 2016, 590, 1791-1803.